Summary
The prevalence of vascular remodeling in hypertension and its relationship to obesity and metabolic syndrome have become more apparent in recent years. Mounting evidence has revealed new mechanisms of vascular remodeling and potential treatment options. This article discusses these new mechanisms and how new evidence may be applied to the management of hypertension and metabolic syndrome.
- Cardiometabolic Disorder
- Hypertensive Disease
- Inflammatory Disease
The prevalence of vascular remodeling in hypertension and its relationship to obesity and metabolic syndrome have become more apparent in recent years. Mounting evidence has revealed new mechanisms of vascular remodeling and potential treatment options. Ernesto L. Schiffrin, MD, PhD, McGill University, Montreal, Quebec, Canada, discussed these new mechanisms and how new evidence may be applied to the management of hypertension and metabolic syndrome.
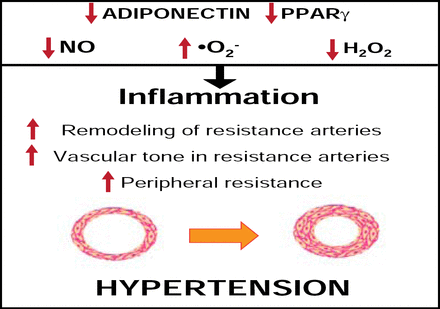
Small artery remodeling may be an early warning sign in essential hypertension and often precedes target organ damage. These structural alterations may also be followed by complications, such as endothelial dysfunction due to oxidative stress and cardiac hypertrophy in some patients [Park JB & Schiffrin EL. J Hypertens 2001]. The degree of structural remodeling and subsequent endothelial dysfunction may be predictive of events in hypertensive patients. Therefore, early detection of structural remodeling and corrective measures to offset any potential deleterious effects may provide more favorable outcomes in this cohort (Figure 1) [Schiffrin EL. Am J Hypertens 2004].
Mechanisms Wherein Metabolic Factors Influence Vascular Remodeling.
Reproduced with permission from E. Schiffrin, MD, PhD.
Characteristics that are associated with the metabolic syndrome may also contribute to vascular dysfunction. In a study by Marchesi and colleagues that investigated oxidative stress and vascular remodeling in a rodent model of metabolic syndrome, New Zealand obese (NZO) mice demonstrated significantly higher media:lumen ratios and media cross-sectional areas within the mesenteric arteries compared with control (p<0.0001 for both), indicating hypertrophic vascular remodeling. Increased perivascular adipose macrophages and tumor necrosis factor receptor alpha (TNFα) and decreased adiponectin were also observed in NZO mice compared with control. Increased NADPH activity and oxidative stress were also observed in the perivascular tissue of NZO mice (p<0.001) compared with control. NZO mice appeared to lose perivascular adipose tissue anticontractile properties, as they were unaffected by norepinephrine compared with control, which demonstrated decreased vasoconstriction to norepinephrine in the presence of perivascular adipose tissue. These findings suggest that metabolic syndrome is associated with perivascular adipose inflammation and oxidative stress, hypertrophic resistance artery remodeling, and endothelial dysfunction [Marchesi C et al. Hypertension 2009].
These findings were supported by data that investigated inflammation in adipose tissue and the mechanisms of vascular dysfunction among obese human subjects with metabolic syndrome. Increased nitric oxide (NO) bioavailability and vasodilation are influenced by healthy adipose tissue secretions, but this capacity is limited in obese patients. In fact, the loss of this dilator effect was accompanied by an increase in adipocyte area (p<0.01) and immunohistochemical evidence of inflammation, as determined by TNFα measurements (p<0.001), in obese patients with metabolic syndrome compared with healthy subjects. Further evaluation of the dilator effect included the addition of the cytokines TNFα and interleukin-6 to perivascular fat around healthy blood vessels. This application resulted in reduced dilator activity and the obese phenotype in previously healthy samples. Study investigators concluded that adipocytes secrete adiponectin, which is a physiological modulator of vascular tone via promotion of NO bioavailability. Thus, obesity contributes to the development of adipocyte hypertrophy and subsequent hypoxia, inflammation, and oxidative stress due to the loss of anticontractile capacity in perivascular fat [Greenstein AS et al. Circulation 2009].
Immune cells, such as T lymphocytes, may also play a role in vascular inflammation and hypertension. Guzik and colleagues demonstrated that hypertension increased T lymphocyte production of TNFα, and treatment with a TNFα antagonist resulted in increased vascular superoxide that was caused by angiotensin II and prevented hypertension [Guzik TJ et al. J Exp Med 2007]. In a recent study by Viel and colleagues, the influence of chromosome 2 (chr2) on vascular inflammation was evaluated using normotensive (Brown Norway), hypertensive (Dahl salt-sensitive), and consomic (SSBN2; in which chr2 had been transferred from Brown Norway to Dahl) rat models. Chr2 replacement resulted in reduced blood pressure (BP) levels and enhanced levels of immunosuppressive mediators compared with genetic salt-sensitive hypertensive (Dahl) rats. Chr2 replacement also prevented aortic inflammatory responses and modulated the regulatory T cell transcription factor Foxp3 and the immunosuppressors transforming growth factor (TGF)-β1 and IL-10 (produced by CD4+CD25+ and CD8+CD25+ cells) [Viel EC et al. AJP-Heart Circ Physiol 2010].
As with the Viel study, transferred regulatory T (Treg) cells were found to ameliorate cardiac damage and improve electric remodeling independently of BP-lowering effects in another rodent study by Kvakan. In this study, Treg adoptive transfer prevented angiotensin II-induced increases in plasma cytokines and oxidative stress monocyte/macrophage infiltration. Aldosterone-induced vascular remodeling was also prevented in this cohort [Kvakan H et al. Circulation 2009]. A review of data that were related to T lymphocytes and their role in hypertension supported the idea that the identification of these mechanisms may foster the development of therapies to improve cardiovascular and renal outcomes in the future [Schiffrin EL. Curr Opin Nephrol & Hypertens 2010].
Based on recent evidence, there appears to be a link between hypertension, metabolic syndrome, and vascular remodeling. New data concerning disease mechanisms, such as NO, oxidative stress, and secretory and T cell dysregulation, may aid in the development of new therapies to prevent and manage hypertension in a variety of groups, including those with metabolic syndrome. Further evaluation of these potential therapeutic targets is needed before treatment strategies can be implemented in clinical practice.
- © 2010 MD Conference Express