Summary
Hypertension is a primary mechanism that is responsible for the progression of cardiovascular and renal damage in patients with elevated blood pressure. In particular, functional and structural changes in the vessel wall may represent the key pathological event in the development of hypertension. Within this disease model, prehypertension may be a marker for subclinical vascular disease. This article reviews evidence that supports the treatment of patients with prehypertension.
- Hypertensive Disease
- Prevention & Screening
Hypertension is a primary mechanism that is responsible for the progression of cardiovascular and renal damage in patients with elevated blood pressure (BP). In particular, functional and structural changes in the vessel wall may represent the key pathological event in the development of hypertension. Within this disease model, prehypertension may be a marker for subclinical vascular disease. In this session, Carlos M. Ferrario, MD, Wake Forest University School of Medicine, Winston-Salem, North Carolina, USA, reviewed evidence that supports the treatment of patients with prehypertension, which is defined as a systolic BP (SBP) from 120 mm Hg to 139 mm Hg or a diastolic BP (DBP) from 80 mm Hg to 89 mm Hg.
The Trial of Preventing Hypertension (TROPHY) was designed to evaluate whether early treatment of patients with high-normal BP levels could slow or prevent the development of hypertension (≥140/90 mm Hg) [Julius S et al. N Engl J Med 2006]. In TROPHY, 809 patients with prehypertension were randomly assigned to treatment with the angiotensin receptor blocker (ARB) candesartan at a dose of 16 mg or a matching placebo for 2 years; then, all patients were treated with placebo for an additional 2 years. Data for analysis were available for 772 patients at the end of the study. Compared with those in the placebo group, patients in the candesartan group were 66.3% less likely to progress to stage 1 hypertension after 2 years (p<0.001) and 15.6% less likely to develop hypertension after 4 years (p<0.007) [Julius S et al. N Engl J Med 2006].
In the Prevention of Hypertension with the Angiotensin-Converting Enzyme Inhibitor Ramipril in Patients with High-Normal Blood Pressure (PHAROA) study, ramipril reduced the 3-year risk of progression from prehypertension to hypertension by 34.4% compared with placebo (42.9% vs 30.7%; p=0.0001) [Lüders S et al. J Hypertens 2008]. Together, findings from TROPHY and PHAROA indicate that a large proportion of patients with untreated high-normal BP will develop hypertension within 2 to 3 years. Moreover, treatment with a renin-angiotensin-aldosterone system (RAAS) inhibitor significantly reduced the risk of progressing to hypertension within the follow-up period that was studied [Julius S et al. N Engl J Med 2006; Lüders S et al. J Hypertens 2008].
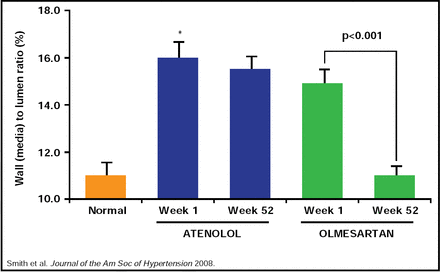
Studies of RAAS blockade in patients with established hypertension demonstrate that it is possible to slow or even reverse vascular damage that is associated with elevated BP. In the Vascular Improvement with Olmesartan Study (VIOS), treatment with an ARB provided greater reversal of vascular hypertrophy compared with beta blockade after 1 year in patients with essential hypertension (Figure 1) [Smith RD et al. J Am Soc Hypertens 2008]. The improvements in vascular resistance were independent of the magnitude of BP reduction that was achieved by ARB or β-blocker therapy.
Reversal of Vascular Hypertrophy with Olmesartan Versus Atenolol in Patients with Prehypertension.
Reproduced with permission from C. Ferrario, MD.
The Multicentre Olmesartan Atherosclerosis Regression Evaluation (MORE) study was designed to compare the effects of olmesartan and atenolol on the progression of atherosclerotic plaques in 165 patients with hypertension and established atherosclerosis [Stumpe KO et al. Ther Adv Cardiovasc Dis 2007]. Patients were randomly assigned to treatment with olmesartan or atenolol for 2 years, and ultrasound evaluations were performed at 28, 52, and 104 weeks to monitor the progression of atherosclerosis and changes in common carotid intima-media thickness (CC-IMT) and plaque volume (PV). Olmesartan and atenolol produced similar reductions in blood pressure levels and CC-IMT at Weeks 28, 52, and 104. Atenolol was associated with a steady, but nonsignificant, increase in PV compared with baseline at each follow-up evaluation. In contrast, olmesartan significantly reduced PV compared with baseline levels at each time period, including 28 weeks (p=0.044), 52 weeks (p=0.038), and 104 weeks (p=0.014), and significantly reduced PV compared with atenolol at 52 weeks (p=0.032) and 104 weeks (p=0.023) [Stumpe KO et al. Ther Adv Cardiovasc Dis 2007].
The VIOS and MORE trials reinforce the role of RAAS inhibitors in reducing structural damage in patients with hypertension. New models of the vascular disease continuum, which note the onset of vascular remodeling prior to the development of hypertension, highlight the potential benefits of the RAAS blockade in patients with prehypertension as well.
- © 2010 MD Conference Express