Summary
The Penumbra System is a mechanical clot removal device that is indicated for the revascularization of occluded large vessels in acute ischemic stroke. It has been approved for commercial use in the United States and Europe. In a postmarket study to assess its safety and effectiveness in a “real world” setting, the performance of the device was deemed to be comparable with the results of the pivotal Penumbra trial with regard to revascularization rate, incidence of adverse events, intracerebral hemorrhage, all-cause mortality, and 90-day good functional outcome.
- Neurology Clinical Trials
- Cerebrovascular Disease
- Ischemia
- Interventional Techniques & Devices
The Penumbra System is a mechanical clot removal device that is indicated for the revascularization of occluded large vessels in acute ischemic stroke (AIS). It has been approved for commercial use in the United States and Europe. In a postmarket study to assess its safety and effectiveness in a “real world” setting, the performance of the device was deemed to be comparable with the results of the pivotal Penumbra trial with regard to revascularization rate, incidence of adverse events, intracerebral hemorrhage (ICH), all-cause mortality, and 90-day good functional outcome.
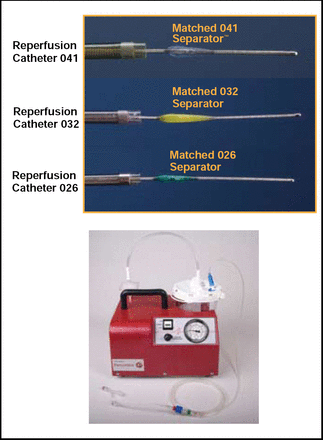
The Penumbra System consists of a reperfusion catheter that is optimized for navigation and aspiration and a separator that cleans and clears clots from the occluded vessel (Figure 1). This was a retrospective case review study of 139 patients who were treated with the device at 7 centers. The primary endpoints were the rate of revascularization of the target vessel (TIMI 2 or 3) and procedural serious adverse events (SAEs). Secondary endpoints included percentage of patients with modified Rankin Score (mRS) ≤2 at 90 days, symptomatic ICH (sICH), and all-cause mortality. Eligible patients had an NIHSS score >8, symptom onset within 8 hours, and TIMI score of 0 or 1 at presentation.
Penumbra Aspiration System.
Mean age of the patient population (72 men and 67 women) was 64±15 years. Mean NIHSS score was 16±6. Target vessels included 26% internal carotid artery (ICA), 51% middle cerebral artery (MCA), and 24% vertebrobasilar artery. This compared with 18% ICA, 70% MCA, and 8% vertebrobasilar artery in the pivotal trial. Arterial puncture was initiated within 4.5 hours from symptom onset, and mean time for revascularization was 48 minutes, similar to that in the pivotal trial.
After use of the Penumbra System, 84% of the treated vessels were revascularized to TIMI 2 or 3. ICH rates at 24 hours were 5.8% and 7.2% for asymptomatic ICH and symptomatic ICH, respectively, which were lower, but not significantly, compared with those in the pivotal trial. There were a total of 8 SAEs and 2 device malfunctions (none of which resulted in patient injury). All-cause mortality was 22% (31/139). At discharge, 34% of the patients had a ≥10 point NIHSS improvement or NIHSS score 0–1. Of the patients who had reached the 90-day follow-up, 40% had a mRS ≤2.
Overall patients in the postmarket study had slightly higher revascularization rates (84% vs 82%), procedural SAEs (5.8% vs 3%), and mRS ≤2 scores (40% vs 25%), while patients in the pivotal study had higher rates of sICH (11% vs 7.2%) and all-cause mortality (33% vs 22%). These results suggest that the postmarket experience of the Penumbra System is consistent with that from the preapproval pivotal trial. Follow-up for some patients is ongoing.
- © 2009 MD Conference Express