Summary
Results from an FDA-approved, prospective pilot trial suggest that the use of a self-expanding, intracranial stent for acute stroke may achieve high levels of revascularization. This follows on the heels of the Mechanical Embolus Removal in Cerebral Ischemia [MERCI; NCT00318071] trials that reported recanalization rates that ranged from approximately 60% to 70% percent with low associated morbidity.
- Cerebrovascular Disease Clinical Trials
- Interventional Techniques & Devices
Results from an FDA-approved, prospective pilot trial that were presented by J. Duffy Mocco, MD, University of Buffalo Neurosurgery, Buffalo, NY, suggest that the use of a self-expanding, intracranial stent for acute stroke may achieve high levels of revascularization. This follows on the heels of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI; NCT00318071) trials that reported recanalization rates that ranged from approximately 60% to 70% percent with low associated morbidity.
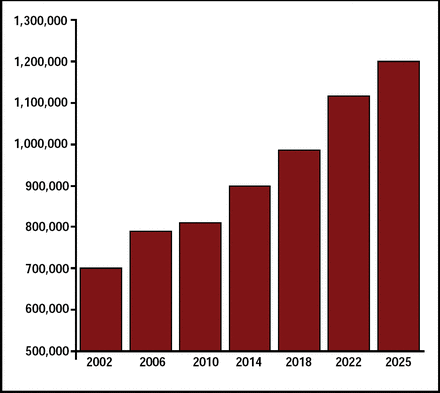
Stroke is a leading cause of long-term disability and the third-leading cause of death. It is estimated that 795,000 strokes occur each year in the United States. This number is projected to increase 40% by the year 2025 (Figure 1). The MERCI 1 [Gobin YP et al. Stroke 2004] and Multi MERCI [Flint AC et al. Stroke 2007] trials, which used the MERCI Retrieval System, compared outcomes in patients who received mechanical embolectomy (recanalization) with outcomes in patients who were not recanalized. Significantly improved clinical outcomes and reduced mortality were reported for the recanalized patient group.
Projected Number of Strokes in the US: 2002–2025.
Although mechanical recanalization appears to work, there are patients for whom the MERCI device fails. Based on the results of this pilot study, Dr. Mocco said he believes that the use of intracranial stenting for acute ischemic stroke after failed thrombolysis with other means is now possible.
Based on preliminary results [Levy EI et al. Neurosurgery 2006; Zaideat OO et al. Stroke 2008] that have demonstrated enhanced outcomes that are produced by self-expanding stents for recanalization of acute cerebrovascular occlusions compared with other means of thrombolysis, the FDA approved the SAIS (Stent-Assisted recanalization in acute Ischemic Stroke) study.
Eligibility included age ≥18 years and presentation within 8 hours of stroke onset. Subjects were required to have an NIHSS ≥8, angiographic demonstration of focal intracerebral artery occlusion not >14 mm, and either contraindication to IV tPA or failure to improve 1 hour after tPA administration. Patients who had CT perfusion imaging that demonstrated >1/3 at-risk territory with nonsalvageable brain or with an intracerebral hemorrhage were excluded. More than 50% of the patients were female, mean age was 63±18 years, mean NIHSS score=14±3.8, and 85% had thrombolysis in myocardial infarction (TIMI)* scores=0.
All 20 patients achieved recanalization; 60% achieved a TIMI score=3 (full flow restored) and 40% had a TIMI score=2 (p<0.0001 compared with presenting TIMI scores). Improvement in NIHSS was documented in 85% of patients, wherein 65% improved by ≥4 NIHSS points. Median NIHSS improvement from intervention to discharge was 9 (range −6 to 14; p<0.001). There were 5 (25%) deaths at 1 month, which compares well with other similar studies. Dr Mocco completed his presentation with a case study of a 65-year-old male with stroke onset 6 hours before presentation and an NIHSS score of 14. Recanalization was achieved in 24 minutes. Four hours after the procedure, the patient's NIHSS score was 0. Based on these data, the FDA has approved an additional 20-patient extension to continue this prospective study, with the movement toward a definitive trial on the horizon.
Notes
-
↵* In this case, TIMI represents the degree of occlusion.
- © 2009 MD Conference Express