Summary
This article data from a post hoc analysis of the Effect of rosuvastatin in patients with chronic heart failure [GISSI-HF; NCT00336336] study, showing only modest evidence of a reduction in atrial fibrillation (AF) in heart failure (HF) patients who were treated with rosuvastatin. The aim of the subanalysis was to assess the effect of n-3 PUFA and rosuvastatin compared with placebo in patients with chronic HF who were not in AF at study entry.
- Arrhythmias
- Heart Failure Clinical Trials
Professor Aldo Maggioni, MD, ANMCO Research Center, Florence, Italy, presented data from a post hoc analysis of the GISSI-HF (Effect of rosuvastatin in patients with chronic heart failure; NCT00336336) study, showing only modest evidence of a reduction in atrial fibrillation (AF) in heart failure (HF) patients who were treated with rosuvastatin. The aim of the subanalysis was to assess the effect of n-3 PUFA and rosuvastatin compared with placebo in patients with chronic HF who were not in AF at study entry.
GISSI-HF was a double-blind, placebo-controlled trial in patients with chronic HF. Patients were randomized to daily treatments of n-3PUFA (1 g) or placebo (n=6975), and to rosuvastatin (10 mg) or placebo (n=4574). Patients were followed for nearly 4 years. Primary endpoints were all-cause mortality or cardiovascular (CV) hospitalizations. The study comprised men and women aged 18 years or older with clinical evidence of HF New York Heart Association class II-IV. Left ventricular ejection fraction (LVEF) was measured within 3 months of enrollment. AF occurrence was defined as the presence of AF on the electrocardiogram (ECG) that was performed at each visit during the trial, AF as a cause of worsening HF, hospital admission, or as an event during hospitalization.
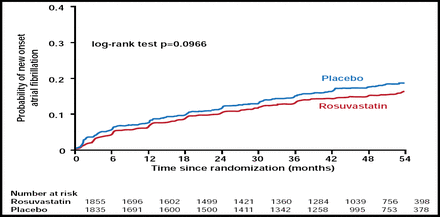
Of the patients without AF at baseline, a total of 15.0% developed AF during a median follow-up of 3.7 years. AF occurred in 16% of the placebo and 13.9% of rosuvastatin patients with a 13.2% RRR and 2.1% absolute risk reduction. This difference was not significant when an unadjusted analysis (p=0.097; Figure 1) or multivariable analysis that adjusted just for clinical variables (p=0.067) was performed. However, it became significant when an adjustment was made for clinical variables and laboratory examinations (p=0.039) and for clinical variables, laboratory examinations, and background therapies (p=0.038).
Kaplan-Meier Curves for Time to New Onset of AF.
Maggioni A et al. Eur Heart J 2009. By permission of Oxford University Press.
Patients who experienced AF during the study were significantly (p≤0.03) older (aged >70 years) and had higher BMI, systolic blood pressure, heart rate, NYHA class, and percent LVEF than those who did not experience an AF. They also had significantly (p<0.05) higher frequencies of prior admission for HF, previous stroke, history of hypertension, pacemaker, history of paroxysmal AF, chronic obstructive pulmonary disorder, and more drug treatment.
Although this post hoc analysis showed some evidence of rosuvastatin's superiority over placebo in reducing the occurrence of AF, it should be noted that the trial was not powered to assess the effect of rosuvastatin on AF occurrence. The effect of a statin treatment that is conducted for a longer period of time or in a larger population of patients should be evaluated to confirm the findings of our study.
The discussant, Professor Harry Crijns, MD, Maastricht University Medical Centre, Maastricht, The Netherlands, agreed that rosuvastatin was not very effective in preventing incidence of AF in this study and suggested that there are a number of unanswered questions, including “whether statins prevent AF progression and reduce the burden of AF” and “whether prevention of AF by statins improves CV morbidity/mortality.”
Full article available at: http://eurheartj.oxfordjournals.org/cgi/content/full/ehp357.
- © 2009 MD Conference Express