Summary
Results from the Randomized Evaluation of Long-Term Anticoagulant Therapy [RE-LY] trial showed that the oral direct thrombin inhibitor dabigatran is a safe and effective alternative to warfarin for the prevention of stroke in patients with atrial fibrillation.
- Cardiology Clinical Trials
- Arrhythmias
- Cerebrovascular Disease
Results from the RE-LY (Randomized Evaluation of Long-Term Anticoagulant Therapy) trial, presented by Professor Stuart Connolly, MD, McMaster University, Hamilton, Ontario, Canada, at the European Society of Cardiology Meeting in Barcelona, Spain, show that the oral direct thrombin inhibitor dabigatran is a safe and effective alternative to warfarin for the prevention of stroke in patients with atrial fibrillation (AF).
RE-LY (NCT00262600) was a phase 3, multicenter, multinational, noninferiority trial that was conducted to compare the efficacy and safety of two different doses of dabigatran with warfarin therapy. The study enrolled 18, 113 subjects (mean age 71 years; 64% men; 50% vitamin K antagonist experienced; mean CHADS2 score 2.1) with electrocardiography-documented nonvalvular AF and at least one of the following: previous stroke or transient ischemic attack, left ventricular ejection fraction <40%, New York Heart Association class ≥II within 6 months before screening, and age ≥75 years (65 to 74 years for subjects with diabetes, hypertension, or coronary artery disease). Subjects were randomly assigned to receive dabigatran 150 mg (n=6076) or 110 mg (n=6015) twice daily in a blinded fashion or open-label, adjusted-dose warfarin (n=6022). Median follow-up was 2 years and complete in 99.9% (20 subjects lost to follow-up). The primary efficacy outcome was hemorrhagic/nonhemorrhagic stroke or systemic embolism, and the primary safety outcome was major hemorrhage.
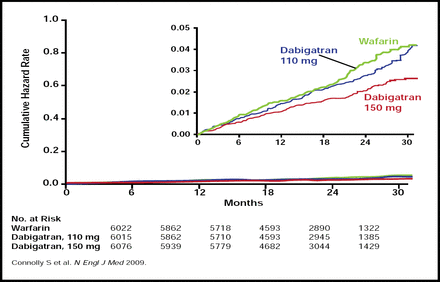
Dabigatran 150 mg twice daily was superior to warfarin in reducing the primary efficacy endpoint (134 subjects; 1.11% per year versus 199 subjects; 1.69% per year; RR, 0.66; 95% CI, 0.53 to 0.82; p<0.001). The risk of major bleeding was similar (3.11% versus 3.36% per year in the dabigatran 150 mg and warfarin groups, respectively; RR, 0.93; 95% CI, 0.81 to 1.07; p=0.31). Meanwhile, dabigatran 110 mg twice daily achieved a similar rate of the primary efficacy endpoint compared with warfarin (182 subjects; 1.53% per year versus 199 subjects; 1.69% per year; RR, 0.91; 95% CI, 0.74 to 1.11; p=0.34; Figure 1), meeting the criteria for noninferiority (p<0.001 for the prespecified noninferiority margin of 1.46), while the rate of major bleeding was significantly lower (2.71% vs 3.36% per year; RR, 0.80; 95% CI, 0.69 to 0.93; p=0.003). The rate of hemorrhagic stroke was significantly (p<0.001) lower with both doses of dabigatran (0.12% and 0.10% per year dabigatran 110 mg and 150 mg, respectively) versus warfarin (0.38% per year).
Cumulative Hazard Rates for the Primary Outcome of Stroke or Systemic Embolism, According to Treatment Group.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
Overall, the mean percentage of time in the therapeutic range for subjects who were randomized to warfarin was 64%. At 2 years, study drug was discontinued in 21% of those who were randomized to dabigatran compared with 16.6% in those who were randomized to open-label warfarin. Adverse events were similar between groups except for dyspepsia, which was significantly more common with dabigatran (707 subjects [11.8%] and 688 subjects [11.3%] in the 110-mg and 150-mg dabigatran groups, respectively, versus 348 subjects [5.8%] in the warfarin group; both p<0.001 compared with warfarin). Importantly, there was no significant difference in rates of abnormal liver function tests between groups, as had been observed with a prior oral direct thrombin inhibitor (ximelagatran).
The net clinical benefit (a composite of stroke, systemic embolism, pulmonary embolism, myocardial infarction, death, or major bleeding) was significantly lower with the 150-mg dose of dabigatran compared with warfarin (RR, 0.91; 95% CI, 0.82 to 1.00; p=0.04) but was not different between the two doses of dabigatran (RR, 0.98; 95% CI, 0.89 to 1.08; p=0.66) or between the 110-mg dose of dabigatran and warfarin (RR, 0.92; 95% CI, 0.84 to 1.02; p=0.10).
- © 2009 MD Conference Express