Summary
Ticagrelor significantly reduced the risk of cardiovascular events and death without increasing major bleeding compared with clopidogrel in patients with acute coronary syndrome, according to findings from the Study of Platelet Inhibition and Patient Outcomes [PLATO; NCT00391872].
- Coronary Artery Disease Clinical Trials
- Myocardial Infarction
Ticagrelor significantly reduced the risk of cardiovascular (CV) events and death without increasing major bleeding compared with clopidogrel in patients with acute coronary syndrome (ACS), according to findings from the Study of Platelet Inhibition and Patient Outcomes (PLATO; NCT00391872).
Ticagrelor is an investigational oral antiplatelet agent that directly and reversibly inhibits the adenosine diphosphate receptor P2Y12. Professor Lars Wallentin, MD, PhD, Uppsala Clinical Research Center, Uppsala, Sweden, reported findings from PLATO, which was designed to evaluate whether ticagrelor is superior to clopidogrel -currently a component of standard therapy for ACS -in preventing vascular events and death in a broad population of patients.
PLATO randomized 18,624 patients who were hospitalized with ACS with or without ST-segment elevation to ticagrelor (180-mg loading dose, 90 mg twice-daily thereafter) or clopidogrel (300-mg to 600-mg loading dose, 75 mg thereafter) in a double-blinded fashion and treated for up to 12 months. All patients were treated with background therapy of aspirin 75 to 100 mg/day. The primary efficacy endpoint was a composite of CV death, myocardial infarction (MI), or stroke. The primary safety endpoint was major bleeding as defined by the trial.
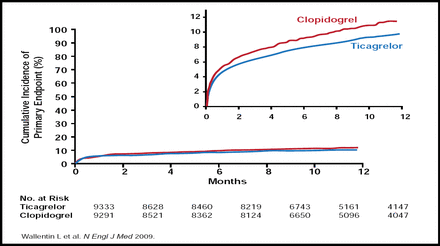
At 12 months, ticagrelor reduced the primary endpoint from 11.7% to 9.8% compared with clopidogrel (HR, 0.84; p<0.001; Figure 1). Ticagrelor also reduced the rates of predefined secondary endpoints compared with clopidogrel, including MI (5.8% vs 6.9%; HR, 0.84; p=0.005) and death from vascular causes (4.0% vs 5.1%; HR, 0.79; p=0.001). However, ticagrelor did not prevent stroke (1.5% vs 1.3%; p=0.22).
Cumulative Kaplan-Meier Estimates of the Time to the First Adjudicated Occurrence of the Primary Efficacy Endpoint.
Copyright © 2009 Massachusetts Medical Society. All rights reserved.
There was no significant difference in the rates of trial-defined major bleeding (11.6% with ticagrelor vs 11.2% with clopidogrel; p=0.43) or TIMI major bleeding (7.9% vs 7.7%; p=0.57). Ticagrelor was associated with increased rates of major bleeding that were not related to coronary artery bypass grafting (CABG), the secondary safety endpoint (4.5% vs 3.8%; p=0.03). There was no significant difference in CABG-related bleeding (7.4% vs. 7.9%; p=0.32).
Overall adverse events were similar; however, ticagrelor was associated with more dyspnea (13.8% vs 7.8%; p<0.001). In addition, among patients who underwent Holter monitoring during the first week of treatment (n=2866), ventricular pauses ≥3 seconds were more frequently seen in those who were randomized to ticagrelor (5.8% vs 3.6%; p=0.01). This difference was not seen on repeat Holter at 30 days (2.1% vs 1.7%; p=0.52). Discontinuation due to adverse events occurred more frequently with ticagrelor compared with clopidogrel (7.4% vs 6.0%; p< 0.001).
Results from PLATO were simultaneously published online in the New England Journal of Medicine. In an accompanying editorial, Professor Albert Schömig, MD, Deutsches Herzzentrum, Munich, Germany, highlighted important differences between PLATO and two other pivotal antiplatelet (P2Y12 receptor antagonists) trials: CURE with clopidogrel and TRITON-TIMI 38 with prasugrel. Of the three trials, PLATO was the only one to demonstrate a reduction in all-cause mortality with more potent platelet inhibition, reducing the risk of overall mortality compared with clopidogrel by 22% (4.5% vs 5.9%; p<0.001).
- © 2009 MD Conference Express