Summary
This article discusses the results of the Kyoto HEART Study [NCT00149227], designed to examine the effects of valsartan as an add-on to conventional therapy on morbidity and mortality in uncontrolled hypertensive patients with one or more cardiovascular risk factors.
- Cardiology Clinical Trials
- Hypertensive Disease
Professor H. Matsubara, MD, Kyoto Prefectural University of Medicine, Kyoto, Japan, reported the results of the Kyoto HEART Study (NCT00149227), designed to examine the effects of valsartan as an add-on to conventional therapy on morbidity and mortality in uncontrolled hypertensive patients with one or more cardiovascular (CV) risk factors. Valsartan (up to 160 mg/daily) add-on treatment to improve blood pressure (BP) control prevented more CV events than conventional non-ARB treatment.
The KYOTO HEART study was a multicenter, prospective, randomized, open-label, blinded endpoint study in 3031 high-risk hypertensive (systolic BP ≥140 and/or diastolic BP ≥90 mmHg) Japanese patients (43% female, mean 66 years) with one or more CV risk factors (such as diabetes, smoking habit, lipid metabolism abnormality, a history of ischemic heart disease, cerebrovascular disease or peripheral arterial occlusive disease, obesity (BMI>25), and left ventricular hypertrophy on electrocardiogram). The primary endpoint was a composite of fatal and nonfatal cerebrovascular and CV events (new-onset or recurrence of stroke, new-onset or recurrence of acute myocardial infarction [MI] or angina pectoris, hospitalization due to heart failure, operation of percutaneous coronary intervention [PCI] or coronary artery bypass graft [CABG], new-onset or recurrence of peripheral arterial disease or aortic dissection, transition to dialysis, and doubling of plasma Cr levels).
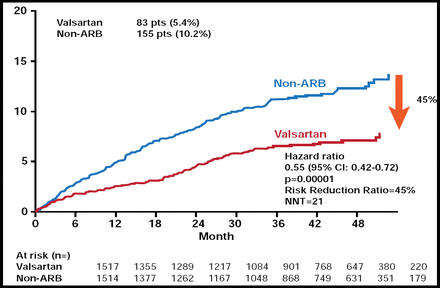
In total, the study gathered information for 8864 patients (valsartan add-on group, 4448; non-ARB group, 4416), and median follow-up period was 3.27 years. After 48 months, the reduction in BP was the same in the two groups (from 157/88 to 133/76 mm Hg). Patients who were treated with valsartan add-on therapy experienced 45% fewer fatal and nonfatal CV events (5.4%) versus those who were on conventional therapy alone (10.2%) (HR, 0.55; 95% CI, 0.42 to 0.72; p=0.00001; Figure 1). There were significant (p<0.03) reductions in the incidences of angina pectoris, stroke, and new-onset diabetes. Adverse events were low in both groups.
Primary Endpoint.
Reproduced with permission by H. Matsubara.
Valsartan add-on treatment to improve BP control prevented more CV events than conventional non-ARB treatment in high-risk hypertensive patients in Japan. These benefits can not be entirely explained by a difference in BP control. Prof. Matsubara believes “this study provides useful information for daily clinical practice in Asian and probably in Europe/US patients.”
The Kyoto HEART study was discussed by Professor F. Ruschitzka, MD, University Hospital, Zurich, Switzerland, who pointed out that the achievement of the primary endpoint in this study was driven by reductions in angina and stroke, but no benefit was seen in the reduction of MI incidence, in which most cardiologists are interested. He cautioned that there is even some evidence (VALUE trial) that valsartan increases the risk of MIs [Julius S et al. Lancet 2004]. Prof. Ruschitzka concluded that ARBs are efficacious and even superior to other drug classes in stroke prevention, but their efficacy with regard to coronary events remains uncertain.
Full article available at: http://eurheartj.oxfordjournals.org/cgi/content/full/ehp363.
- © 2009 MD Conference Express