Summary
Results from the FRANCE (French Aortic National CoreValve and Edwards) Registry demonstrated high implantation success with excellent and sustained hemodynamic and clinical improvement in high-risk patients with severe aortic stenosis who were treated with transcatheter aortic valve replacement.
- Cardiology Clinical Trials
- Valvular Disease
- Interventional Techniques & Devices
Results from the FRANCE (French Aortic National CoreValve and Edwards) Registry, presented by Helene Eltchaninoff, MD, University of Rouen, Rouen, France, demonstrated high implantation success with excellent and sustained hemodynamic and clinical improvement in high-risk patients with severe aortic stenosis who were treated with transcatheter aortic valve (TAVI) replacement.
TAVI is an emerging treatment for patients with aortic stenosis who are at too high a risk to undergo conventional surgical replacement of the aortic valve. The FRANCE Registry is a multicenter prospective clinical registry that was developed to evaluate the safety and efficacy of the two aortic valve replacement devices that are currently available in France. The two valves that were used in the registry were the Edwards Sapien balloon-expandable valve (68% of patients), using either a transfemoral (39%) or transapical (29%) approach, and the CoreValve self-expandable valve (32% of patients), using a transfemoral (27%) or subclavian approach (5%).
Study patients were required to have severe aortic stenosis (effective orifice area [EOA] <1 cm2/m2) and severe symptoms (New York Heart Association [NYHA] Class ≥2) and be at high surgical risk (Logistics EuroScore >20%, Society of Thoracic Surgeons [STS] mortality risk score >10%), or have a contraindication to surgery). The primary endpoint of the study was 30-day mortality. Secondary endpoints (up to 3 years) included mortality, major adverse cardiac events, hemodynamics, and quality of life.
A total of 244 patients (mean age 82 years; 56% men) were recruited between February and September 2009. Diabetes was present in 27% patients; 23% had a previous myocardial infarction; 10% had a previous stroke; and ∼42% had coronary artery disease. The only significant (p=0.02) difference between the four subgroups was the presence of peripheral artery disease and abdominal aortic aneurysm, which were more common in patients who were treated with a transapical or subclavian approach. The mean baseline EuroScore was 25.6%; mean STS score was 16%. The mean aortic annulus (21.9±1.8 mm) was slightly smaller in patients who received the Edwards due to the availability of the 23-mm stent and larger in the CoreValve group due to the availability of a 29-mm stent. The mean EOA was 0.68±0.16 cm2. Mean left ventricular ejection fraction was 51% (47% [p=0.02] in patients who received the Edwards valve via the transfemoral approach). Two-thirds of the procedures were done in the cardiac catherization lab.
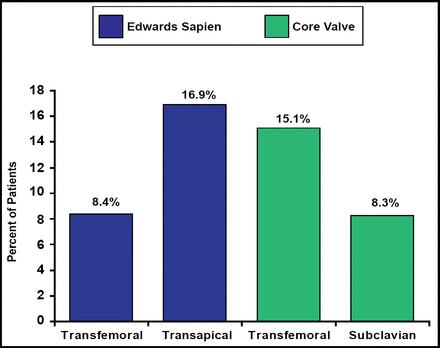
The devices were successfully implanted (defined as successful delivery and deployment of the valve without death on the table) in 97% of patients. Failure occurred in 7 patients; there were 4 procedural deaths. There was no difference between the groups in 30-day mortality (mean 12.7%; p=0.32; Figure 1). Hemodynamic results immediately after implantation were significant (mean increase in EOA from 0.68±0.16 cm2 to 1.74±0.47 cm2; p<0.001). The rate of new pacemaker implantation (overall mean 11.8%) was significantly (p<0.001) higher in the CoreValve group (25% to 27%) compared with 4% to 5% in the Edwards valve group. The transfusion rate (mean 21.3%) was higher when a transapical (27.4%) or subclavian (83.3%) approach was used.
30-Day Mortality.
Reproduced with permission by H. Eltchaninoff, MD.
Vascular complications (mean 6.5% of patients) were comparable between the four groups. Postimplantation aortic regurgitation occurred in <10% of patients. Factors that were predictive of 30-day mortality (by univariate and multivariate analysis) were prior CABG and Euroscore ≥25%.
A total of 111 patients have reached the 6-month follow-up. Survival at 6 months is 76.5%. Hemodynamic and clinical results are persistent.
- © 2009 MD Conference Express