Summary
Data from studies such as the Surgical Trial in Intracerebral Hemorrhage [STICH] and Factor Seven for Acute Hemorrhagic Stroke Treatment [FAST] trial have suggested that intraventricular hemorrhage (IVH) volume is a predictor of mortality. Preclinical data suggest that intraventricular thrombolysis leads to faster ventricular clearance and better recovery. The CLEAR- IVH trial sought to follow up on these ideas in a phase 2 study, and this article gives an overview of the results and plans for a phase 3 study.
- neurology clinical trials
- interventional techniques & devices
- ischemia
Data from studies such as the Surgical Trial in Intracerebral Hemorrhage (STICH) and Factor Seven for Acute Hemorrhagic Stroke Treatment (FAST) trial have suggested that intraventricular hemorrhage (IVH) volume is a predictor of mortality. Preclinical data suggest that intraventricular thrombolysis leads to faster ventricular clearance and better recovery. The CLEAR- IVH trial sought to follow up on these ideas in a phase 2 study. The study completed enrollment at the end of 2007, and Issam Awad, MD, Northwestern University, Evanston, IL, gave an overview of the results and plans for a phase 3 study.
The CLEAR-IVH study enrolled patients aged 18–75 years with IVH within 12 hours of symptom onset who had extraventricular drainage per medical management. Patients with infratentorial intracerebral hemorrhage (ICH), surpratentorial ICH>30 cc, unclipped aneurysm, or active internal bleeding; who were on heparin >10,000 U/day or coumadin; or who had major medical conditions were excluded. Once they were considered stable (ie, after 6 hours and repeat CT that demonstrated no clot enlargement), patients gave informed consent and were assigned in a 1:1 ratio to either recombinant tissue plasminogen activator (rt-PA) or placebo. Dose escalation was subsequently performed in three other tiers: 0.3 mg Q12h, 1.0 mg Q12h, and 1.0 mg Q8h. If the investigator believed it was necessary, a second external ventricular drain (EVD) was allowed in cases of a trapped ventricle or intractable intracranial pressure.
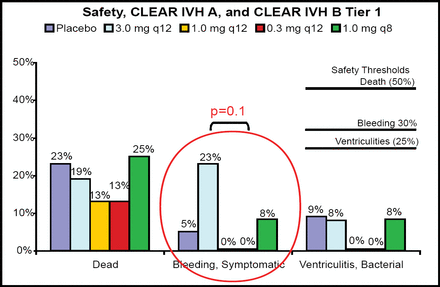
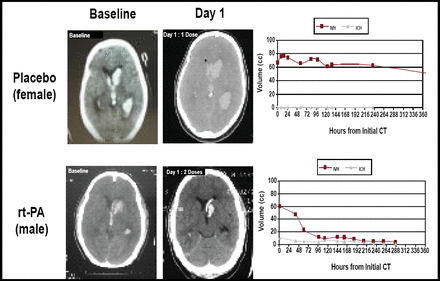
Twenty-two patients were treated with placebo and 88 with rt-PA. The bleeding threshold of 30% was never approached in any tier (Figure 1). “Cases treated with rt-PA had very dramatic clearance of the ventricles,” said Dr. Awad (Figure 2). The entire cohort demonstrated a significantly faster clearance when treated with rt-PA compared with placebo (p<0.05). There was no relationship between dose group and overall clearance; however, there was a significant relationship between dose and clearance of the third and fourth ventricles (p=0.01). Modified Rankin scale data displayed a trend toward better outcomes in the treated group at 30, 90, and 180 days post-injury. Patients with rt-PA treatment had a lower number of treatment days (7.5 vs 12). For the safety phase (initial 48 subjects), only one rt-PA patient needed three EVDs, as opposed to seven patients in the placebo group.
10 Endpoint Comparison: Safety, CLEAR A & B –IVH Trials.
Comparison Treatments: Baseline and Day 1.
When examining data from large clots in trapped ventricles, placement of a second catheter led to better clearance. “This clotbuster works, but you have to put the catheter in the right place,” commented Dr. Awad. Based on the second catheter data, the surgeon's committee suggested that dual catheters be considered for large IVH, and that image-guided placement of the second catheters be utilized. A large phase 3 randomized, placebo-controlled trial of 500 subjects has been planned and will hopefully start later this year. For more information regarding this clinical program, visit www.clearivh.com.
- © 2008 MD Conference Express