Summary
Argatroban is a direct thrombin inhibitor that safely augments the benefit of tPA in animal stroke models; however, human data are limited. The Argatroban tPA Stroke Study [ARTSS; NCT00268762] is a multicenter, Phase II, prospective, open-label, safety and activity study of argatroban and tPA in patients with ischemic stroke.
- Neurology
- Ischemia Clinical Trials
The benefit of intravenous recombinant tissue plasminogen activator (tPA) in acute ischemic stroke is related to clot lysis and arterial recanalization. Argatroban is a direct thrombin inhibitor that safely augments the benefit of tPA in animal stroke models; however, human data are limited. The Argatroban tPA Stroke Study (ARTSS; NCT00268762) is a multicenter, Phase II, prospective, open-label, safety and activity study of argatroban and tPA in patients with ischemic stroke. The final results of ARTSS were presented by Andrew D. Barreto, MD, and James C. Grotta, MD, University of Texas-Houston, Houston, Texas, on behalf of the ARTSS investigators.
The primary (safety) outcome for ARTSS was the incidence of significant (ie, symptomatic or PH-2) intracerebral hemorrhage (ICH). Secondary (signal of efficacy) outcomes included rates of recanalization, measured at 2 and 24 hours by transcranial Doppler (TCD) ultrasonography or CT-angiogram, and modified Rankin Scale score at discharge. There was also a preplanned historical control comparison with the control data (IV-rtPA alone) from the CLOTBUST study [Alexandrov AV et al. NEJM 2004].
Eligibility included patients from 18 to 85 years of age who were admitted between 0 and 4.5 hours of stroke onset and met the criteria for intravenous tPA therapy. Subjects were also required to be within the NIH stroke scale (NIHSS) limits of 5–20 on the left hemisphere and 5–15 on the right hemisphere, have a proximal intracranial arterial occlusion that was measured by TCD or CT-angiogram, an INR ≤1.5, and no known hepatic disease. Subjects received full-dose IV-tPA (0.9 mg/kg) plus argatroban, given as a 100-mcg/kg bolus that was started during the tPA infusion and then as a 1-mcg/kg infusion over 48 hours. Argatroban was titrated to a target partial thromboplastin time (PTT)=1.75 by the patient's baseline.
Subjects (n=65) had a mean age of 63 years, and 45% were men. The median subject NIHSS score was 13. Ninety percent of patients had middle cerebral artery occlusions, and the majority of patients were enrolled using TCD (n=47). The median time from the symptom onset to tPA bolus was 128 minutes (interquartile range 94 to 170 minutes). There was a median time of 17 minutes of overlap for the tPA and argatroban.
Target PTT (+10%) was achieved. Significant ICH occurred in 4 subjects (6.2%); three of them were symptomatic hemorrhage (4.6%) and 2 were parenchymal hemorrhage type 2 (PH-2; 3.1%). One patient was symptomatic and had a PH-2. There were 185 adverse events; 28 were considered serious, 3 were likely related to the treatment, and none was considered definitely related to treatment. There were 7 deaths (10.8%) by discharge or Day 7.
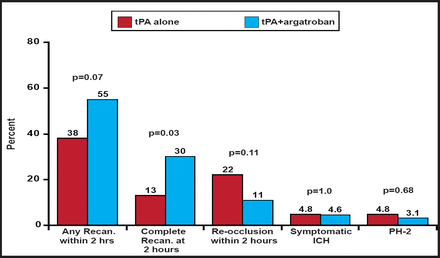
Of the 47 subjects who received TCD, 55% recanalized (complete recanalization in 30% and partial recanalization in 26%) at 2 hours. Five subjects (11%) recanalized before 2 hours but reoccluded at 2 hours. Of the 60 subjects with 24-hour data, 60% had complete recanalization and 18% had partial recanalization.
When compared with the controls from the CLOTBUST study, significantly (p=0.03) more patients in ARTSS achieved complete recanalization at 2 hours (Figure 1). There was also a trend toward reduced reocclusion. No difference in symptomatic ICH was found. A randomized, controlled Phase IIb study is planned to confirm and extend these findings.
Comparison to CLOTBUST Controls.
Reproduced with permission from A. Barreto, MD.
- © 2011 MD Conference Express