Summary
Two ASTEROID and STRADIVARIUS trials explored the potential for pharmacologic therapy to slow the progression of atherosclerosis or even cause plaque regression in patients with coronary artery disease. The studies involved different classes of drugs and different imaging modalities to evaluate the change in the degree of stenosis caused by the atherosclerotic plaque.
- lipid disorders
- coronary artery disease clinical trials
Two trials explored the potential for pharmacologic therapy to slow the progression of atherosclerosis or even cause plaque regression in patients with coronary artery disease (CAD). The studies involved different classes of drugs and different imaging modalities to evaluate the change in the degree of stenosis caused by the atherosclerotic plaque.
In the ASTEROID trial, 507 patients with angiographic evidence of CAD were treated with rosuvastatin 40 mg/day for 24 months in an uncontrolled observational study. The initial results, first presented in 2006, demonstrated that the drug reduced plaque volume, as measured by intravascular ultrasound (IVUS), in arteries with less than 50% stenosis. Rosuvastatin 40 mg was well tolerated, with low rates of elevated ALT (1.8%) and CK (1.2%) observed, and only 12% of patients discontinuing due to adverse events. The current analysis was performed to evaluate changes in vascular lumen by quantitative coronary angiography (QCA) in arteries with more than 25% stenosis.
Christie Ballantyne, MD, Baylor College of Medicine, Houston, TX, reported that the coronary angiograms of 292 patients were evaluated at baseline and at 24 months. QCA showed that treatment with rosuvastatin led to an increase in the mean minimal lumen diameter from 1.65 ± 0.36 mm to 1.68 ± 0.38 mm (p<0.001) and a decrease in the mean percentage diameter stenosis from 37.3 ± 8.4% to 36.0± 10.1% (p<0.001).
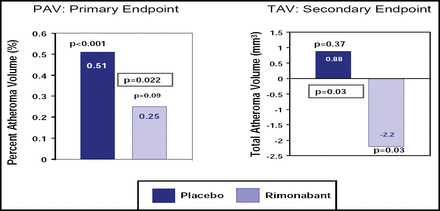
In STRADIVARIUS, 839 abdominally obese patients with CAD were randomly assigned to treatment with either rimonabant 20 mg daily (422 patients) or placebo (417 patients). Rimonabant, a cannabanoid type 1 (CB1) receptor inhibitor, is an experimental agent that is not yet approved in the United States but is available in some European countries. The percent atheroma volume (PAV) and normalized total atheroma volume (TAV) on IVUS were determined in all patients at the beginning of the study and again at 18 months in the 676 patients who completed the trial, regardless of whether they were still taking the study drug.
Steven E. Nissen, MD, Cleveland Clinic, Cleveland, OH, reported that rimonabant (compared with placebo) failed to reduce the primary endpoint of PAV (+0.25% change from baseline with rimonabant vs +0.51% with placebo; p=0.22; Figure 1). However, there was a significant difference between the two groups with respect to the secondary endpoint of change in TAV from baseline (−2.2 mm3 rimonabant vs +0.88 mm3 placebo; p=0.03).
Primary and Secondary IVUS Endpoints.
Of concern in the study was the higher frequency of psychiatric adverse events in the rimonabant arm (43.4% vs 28.4%; p<0.001). This difference was primarily driven by significant increases in anxiety (18.0% vs 11.8%; p=0.01) and depression (16.8% vs 11.3%; p=0.02). Dr. Nissen pointed out that approximately one-quarter of the patients in the study had a history of psychiatric disease at the start of the trial. The risk of psychiatric adverse events was a factor in the unanimous recommendation against approval of the drug by a U.S. Food and Drug Administration advisory panel in 2007.
The lipid-lowering benefits of both rosuvastatin and rimonabant were confirmed by these two studies. Rosuvastatin reduced low-density lipoprotein (LDL) levels by more than 50% and increased high-density lipoprotein (HDL) levels by 14%, while rimonabant increased HDL by 22% and reduced triglyceride levels by 21%. Dr. Ballantyne noted that it may be necessary to reduce LDL by >50% or set a target LDL near 60 mg/dL to induce plaque regression in coronary atherosclerosis.
The results of the QCA analysis from the ASTEROID trial were published online (Circulation 2008), and the primary results of STRADIVARIUS were published in JAMA 2008;299:1547–1560.
- © 2008 MD Conference Express