Summary
The angiotensin-receptor blocker telmisartan is equally effective in reducing cardiovascular risk as the angiotensin-converting enzyme inhibitor ramipril in patients with vascular disease or high-risk diabetes. However, the combination is no more effective than either drug alone and causes more side effects according to results from the ONTARGET Trial.
- diabetes mellitus
- coronary artery disease clinical trials
The angiotensin-receptor blocker (ARB) telmisartan is equally effective in reducing cardiovascular risk as the angiotensin-converting enzyme (ACE) inhibitor ramipril in patients with vascular disease or high-risk diabetes. However, the combination is no more effective than either drug alone and causes more side effects.
“Physicians and patients now have a choice as to whether to use telmisartan or ramipril,” said Salim Yusuf, MD, McMaster University, Hamilton, Ontario, Canada, principal investigator of ONTARGET “We can use telmisartan with confidence when we believe an ACE inhibitor is not tolerated,” he said. Dr. Yusuf estimated that ACE intolerance affects “at least 20% to 30% of patients.”
ONTARGET enrolled 25,620 patients with coronary heart disease or diabetes plus additional risk factors, but no evidence of heart failure. Patients were randomly assigned to treatment with ramipril 10 mg per day (n=8576), telmisartan 18 mg per day (n=8542), or the combination of ramipril and telmisartan (n=8502).
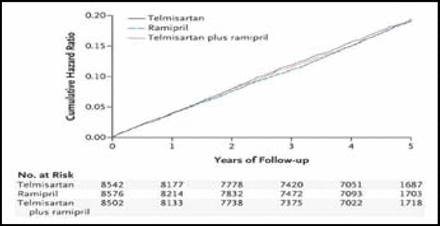
At a median follow-up of 56 months, a similar proportion of patients in each group reached the primary endpoint, a composite of death from cardiovascular causes, myocardial infarction (MI), stroke, or hospitalization for heart failure (Figure 1). Cardiovascular events were observed in 16.5% of patients in the ramipril group, compared with 16.7% in the telmisartan group (RR 1.01; 95% CI, 0.94–1.09) and 16.3% in the combination therapy group (RR 0.99; 95% CI, 0.92–1.07), suggesting that the three regimens were equally effective in preventing adverse cardiovascular outcomes.
Cardiovascular Events with Ramipril, Telmisartan, or Both. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Cough was the most common reason for discontinuation of therapy during ONTARGET (Table 1). Compared with the ramipril group, the telmisartan group had a lower rate of both cough (4.2% vs 1.1%; p<0.001) and angioedema (0.3% vs 0.1%; p=0.01). Patients in the telmisartan group were more likely than those in the ramipril group to report symptoms of hypotension (2.7% vs 1.7%; p<0.001), although both groups had a similar rate of syncope (0.2%). Patients in the combination group were much more likely than the ramipril group to discontinue therapy due to hypotensive symptoms (RR 2.75; p<0.001) and syncope (RR 1.95; p=0.03).
Treatment Discontinuations with Ramipril, Telmisartan, or Both.
Findings for the major secondary outcome, a composite of cardiovascular death, MI, or stroke (modeled after the primary outcome of the Heart Outcomes Prevention Evaluation (HOPE) trial), % of patients in the ramipril group and 13.9% of patients in the telmisartan group (RR 0.99; 95% CI, 0.91–1.07). Combination therapy was not significantly different than ramipril alone (RR 0.99; 95% CI, 0.92–1.07) with respect to this major secondary composite endpoint.
Based on these findings, Dr. Yusuf said that ramipril and telmisartan can be “used interchangeably” in this patient population. However, evidence from ONTARGET suggests that caution should be used if these agents are combined, because side effects were increased, without clear benefit.
Results of ONTARGET were published simultaneously with the late breaking trials session in the New England Journal of Medicine [The ONTARGET Investigators. N Engl J Med 2008;15:1547–59].
- © 2008 MD Conference Express