Summary
This article discusses the latest advances concerning genes and the environment, and their impact on the development of rheumatoid arthritis (RA). Other topics include the need for new treatment strategies for early RA, and results from the RAPID 1 trial and FIN-RACo study, among other things.
Lars Klareskog, MD, Karolinska Institute, Stockholm, updated EULAR attendees on the latest advances concerning genes and the environment, and their impact on the development of rheumatoid arthritis (RA). “RA is a heterogeneous syndrome whose etiology and pathogenesis can be defined by subphenotypes”, said Prof. Klareskog.
In a recent study, smoking (an environmental factor), in the context of HLA-DR shared epitope genes, was identified as a possible trigger for RA-specific immune reactions to citrullinated proteins. Since antibodies against citrullinated proteins are specific and predictive markers for RA, Prof. Klareskog suggested that an etiology involving a definite genotype, an environmental provocation, and the induction of specific autoimmunity, impacts the development of a distinct subset of RA [Klareskog L et al. Arthritis Rheum 2006].
Prof. Klareskog believes that information of this nature will lead to the use of genetic epidemiology as a basis for studies on the molecular pathology of RA, possibly resulting in a new taxonomy built on these different molecular pathologies. New therapies may then be based on the different molecular pathologies that cause RA and tailored to the individual.
The current goals of RA therapy are to produce remission, halt and heal disease related destruction, reverse disability and return the patient to a normal life expectancy. To do this however, therapy needs to be optimized and more information is required to understand the response to treatment of the individual patient. To this end, “new clinical and biological markers are needed that can predict those patients most likely to respond to a particular treatment”, stated Josef S. Smolen, MD, Medical University of Vienna.
New markers such as the cartilage oligometrix matrix protein (COMP), a glycoprotein, are being tested as possible identifiers of joint damage. However, inheritable factors, diurnal variation, and degrees of progression influence serum levels of COMP and can compromise the usefulness of this and other marker.
Effective therapy, such as with prednisolone, is associated with a marked reduction in macrophage infiltration in RA synovial tissue, suggesting that the number of synovial macrophages could be used as a biomarker for clinical efficacy [Gerlag DM et al. Arthritis Rheum 2004]. However, the usefulness of this biomarker, which requires an invasive procedure, still needs to be validated based on demonstrated superiority to markers using other sources, such as serum or clinical markers.
High titer rheumatoid factor and anti-CCP, and especially C-reactive protein (CRP), continue to be the most reliable biomarkers for predicting joint damage. However, a highly reliable predictor for future disease activity are composite disease activity indices. At 3 months from start of treatment, the score of the simplified disease activity index was highly predictive of disease activity at one year. Nevertheless, additional prediction models may be needed to optimize therapy decisions. The combination of clinical and biological markers may allow for RA prediction for the effects of targeted therapies on the level of the individual patient.
Given our improved understanding of the causes and detection of RA, Thomas Huizinga, MD, Leiden University Medical Center, The Netherlands, addressed the need for new treatment strategies for early RA. He empathized that RA should be diagnosed and treated with disease-modifying anti-rheumatic drugs (DMARDs) as early as possible. Delay of DMARD therapy has been shown to be a major contributing factor to poor outcome. There is a window of opportunity for highly successful RA treatment in the first year, and especially within the first 3 months of diagnoses [Nell VP et al. Rheumatology 2004].
Results of the PROMPT study have shown that immediate treatment with MTX retards damage. Undifferentiated arthritis progressed to RA in 40% of the MTX treated patients vs 53% of patients in the placebo group. However, in the MTX group, a significant (p=0.046) time delay for patients fulfilling the ACR criteria for RA was noted compared with the placebo group. In addition, significantly (p=0.046) fewer patients showed radiographic progression (p< 0.05) suggesting that early treatment with MTX delayed the progression of RA [van Dongen H et al. Arthritis Rheum 2007].
Grigor and colleagues have shown that a strategy of intensive outpatient management of RA, as opposed to routine outpatient care, substantially improves disease activity, radiographic disease progression, physical function, and quality of life at no additional cost [Grigor C et al. Lancet 2004]. Prof. Huizinga believes that there is utility in monotherapy, but combination therapy is better. The major challenge is differentiating between patients who should receive MTX monotherapy versus those who should receive combination therapy at disease onset.
The RAPID 1 phase 3 study results, presented by Edward C. Keystone, MD, University of Toronto, reported on the efficacy and tolerability of two dose regimens of certolizumab pegol, the first PEGylated anti-TNF to be studied as add-on therapy in patients with active RA who are refractory to MTX monotherapy. Patients, previously treated for ≥6 months with MTX, were treated with certolizumab pegol, three 400 mg doses every 2 weeks or placebo, followed by certolizumab pegol (200 mg or 400 mg) every 2 weeks, or placebo. MTX therapy was continued as usual. At week 24, the ACR20 response was 59.2% in the certolizumab pegol 200 mg group, 61.2% in the 400 mg group, and 13.5% in the placebo group (MTX alone) (p<0.001 active vs placebo). Significant differences from placebo (p<0.001) were also reported for the ACR50 and ACR70 endpoints.
The majority of adverse events were mild to moderate. Prof. Keystone commented, “when treatment with MTX as a monotherapy does not achieve sufficient results, we have shown that adding certolizumab pegol can achieve significant improvement in symptoms.”
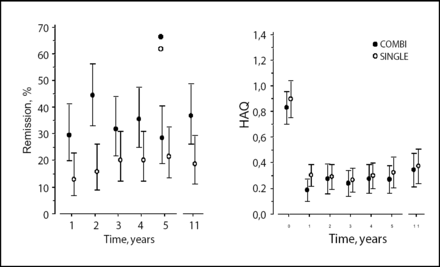
Vappu Rantalaiho, MD, Tampere University Hospital, Finland, presented results of the FIN-RACo study which evaluated the long-term frequency of remission and change in HAQ values over the course of 11 years in patients with early RA initially treated either with a combination of 3 DMARDs or a single DMARD for 2 years. The estimated remission rate over time was 32% in the combination group and 19% in the single group (p=0.0082; Figure 1). The mean improvement in HAQ was not significantly different between the two groups. In the combination group, remission at 6 months was a strong predictor of remission at 11 years – emphasizing the importance of early and aggressive DMARD therapy in RA.
Remission Rates Over Time: Combination vs Single Therapy.
In a phase 2 trial, ofatumumab (HuMax-CD20), a human monoclonal anti-CD20 IgG1 antibody, was administered to patients with active RA. Mikkel Øtergaard, MD, Copenhagen University Hospital, Denmark, reported that a significantly increased proportion of patients receiving 2 infusions of ofatumumab (300 mg, 700 mg or 1000 mg) obtained an ACR20 response at week 24 (41% [p=0.002], 49% [p<0.001] and 46% [p<0.001], respectively) vs patients receiving placebo (15%). Likewise, significantly more patients had good or moderate EULAR responses after treatment with ofatumumab (72% in each dose group) vs placebo (40%; p=0.001).
Ofatumumab was generally well tolerated and all patients tested negative for human anti-human antibodies. Rapid and sustained peripheral CD19+ B-cell depletion was observed in all ofatumumab dose groups. This first analysis of data from an ongoing trial shows encouraging results of ofatumumab therapy in patients with RA.
- © 2007 MD Conference Express