Summary
In 2002, a landmark report from the Women's Health Initiative (WHI) led to a widespread halt in the prescribing of hormone treatment for postmenopausal women when the study showed that serious risks outweighed benefits when the treatment was used for chronic disease prevention. Nine years later, the use of hormone therapy for postmenopausal women remains a complex and challenging topic.
- Hormone Therapy
- Menopause
In 2002, a landmark report from the Women's Health Initiative (WHI) led to a widespread halt in the prescribing of hormone treatment for postmenopausal women when the study showed that serious risks outweighed benefits when the treatment was used for chronic disease prevention. Nine years later, the use of hormone therapy for postmenopausal women remains a complex and challenging topic.
“Many clinicians have decided not to prescribe hormone therapy for their patients,” said JoAnn E. Manson, MD, DrPH, FACE, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA, one of the principal investigators for the WHI. Dr. Manson added that researchers have “learned a lot since 2002” and noted that hormone therapy still has a clinical role in the treatment of moderate-to-severe menopausal symptoms.
“Hormone therapy is neither good nor bad for all women, and it is very clear that there is no one- size-fits-all answer for clinical decision-making,” said Dr. Manson. She pointed to age and time since menopause as major factors that can help identify women who are good or poor candidates for hormone therapy, especially with regard to the risk for coronary heart disease (CHD).
The Women's Health Initiative Trials
The WHI Hormone Therapy Trials were designed to assess the role of menopausal hormone therapy in chronic disease prevention. The WHI Estrogen + Progestin (E+P) Trial was stopped more than 3 years early when it became clear that the risks of treatment—increased risks for breast cancer (26%), CHD (29%), stroke (41%), and pulmonary embolism (113%)—far outweighed the benefits of decreased risks for hip fracture (34%) and colorectal cancer (34%) [Writing Group for the Women's Health Initiative. JAMA 2002]. The WHI Estrogen-Alone (E-alone) trial was also stopped early, with findings of a 39% increased risk for stroke. Although estrogen had a neutral effect on other risks, “it was still not a good tradeoff for chronic disease prevention,” said Dr. Manson.
The WHI findings regarding CHD risks conflicted with the results of previous observational studies of postmenopausal hormone therapy. Dr. Manson said that more than 40 observational studies of hormone therapy had shown that the relative risks of CHD were 40% to 50% lower among current or ever-users of hormone therapy compared with never-users [Grodstein F et al. Prog Cardiovasc Dis 1995]. “This percentage was probably an overestimate of the benefit, but confounding and selection biases that may influence observational studies may not have been the only factors,” said Dr. Manson. She pointed to “very substantial” differences between the study populations in the WHI and the observational cohorts; women in the WHI were a mean of 11 years older (63 vs 52 years), had a longer time since menopause (12 or more vs 1 to 3 years), generally had no vasomotor symptoms (compared with the presence of vasomotor symptoms), and had a higher mean body mass index (28 to 30 vs 24 to 25 kg/m2).
Effect of Age and Time Since Menopause
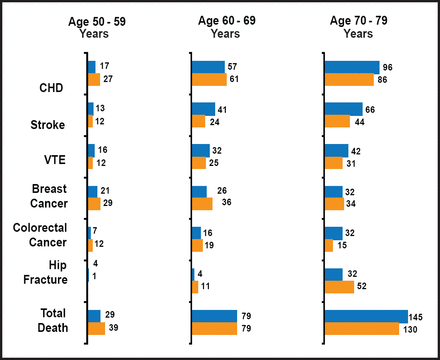
When data from the two initial WHI trials were evaluated according to age group, it became apparent that the absolute rates of adverse events were much lower for younger women than for older women. For example, the E-alone trial, compared with women aged 70 to 79 years, the women who were aged 50 to 59 years had one-quarter to one-fifth of the absolute risks for CHD, stroke, venous thromboembolism, and all-cause mortality, as well as lower risks for colorectal cancer and hip fracture (Figure 1). In terms of relative risks, the E+P trial did not show a clear interaction by age (the relative risk of CHD for E+P vs placebo was 1.27 for women aged 50 to 59 years, 1.05 for women aged 60 to 69 years, and 1.44 for women aged 70 to 79 years). When the results were analyzed by time since the onset of menopause, however, it became clear that the relative risks of CHD, comparing E+P with placebo, increased with longer duration of time since menopause: RR=0.89 for women who were less than 10 years, 1.22 for women who were 10 to 19 years, and 1.71 for women 20 or more years from the onset of menopause [Manson JE et al. N Engl J Med 2003].
Results from the WHI Estrogen-Alone and Health Outcomes Trial (According to Age Group).
Numbers represent number of cases per 10,000 women per year. Blue=estrogen, yellow=placebo. CHD=coronary heart disease, VTE=venous thromboembolism.
Reproduced with permission from J. Manson, MD, DrPH.
Evaluation of data in the E-alone trial showed 30% to 40% reductions in specific cardiac outcomes (myocardial infarction [MI], coronary revascularizations, and a composite of MI plus coronary revascularization) among women aged 50 to 59 years, whereas the risks of these outcomes were neutral or slightly increased in women aged 60 to 69 or 70 to 79 years [Hsia J et al. Arch Intern Med 2006]. Coronary artery calcium levels, a marker for risk of cardiovascular events, were also lower with E-alone, compared with placebo, among the younger women [Manson JE et al. N Engl J Med 2007]. Pooled analysis of data from both initial WHI trials (E+P and E-alone) showed a significant trend in reduction of CHD according to time since menopause. In addition, a 30% reduction in all-cause mortality for women aged 50 to 59 years emerged, compared with increased risks for both CHD and all-cause mortality among women aged 70 to 79 years [Rossouw JR et al. JAMA 2007]. The absolute risks for CHD, stroke, all-cause mortality, and global index (a composite of adverse outcomes) differed significantly according to age (Table 1). The most recent analysis of risk according to age involved a subset of WHI participants who were followed up for 10.7 years to determine the postintervention and cumulative health outcomes with E alone [LaCroix AZ et al. JAMA 2011]. In that study, the relative risks of CHD, MI, all-cause mortality, and the global index were significantly lower among younger women (aged 50 to 59 years) than among older women (aged 70 to 79 years).
Absolute Excess Risks (Cases per 10,000 Person-Years) by Age in the Two Initial WHI Trials.
Dr. Manson said that taken together, studies indicate that very few younger women will have a substantial risk for cardiovascular or other adverse events with short-term hormone therapy. She explained that recent menopause typically corresponds to a time of early stages of atherosclerosis, when estrogen has generally favorable effects on the endothelium and plaque development. However, once advanced atherosclerosis is present, estrogen may have prothrombotic, proinflammatory, and plaque-destabilizing effects that can precipitate plaque rupture. These adverse effects of estrogen support the findings of increasing CHD risk as the time since the onset of menopause increases. Dr. Manson also noted that the observational studies that showed that decreased CHD risks are associated with hormone therapy included primarily women who were less than 5 years from menopause at the time that they initiated hormone therapy, further explaining the conflicting data between these studies and the WHI trial results.
Breast Cancer Risk
The risk of breast cancer that is associated with hormone therapy differs substantially by the type of treatment (E+P vs E-alone). With use for 4 to 5 years, E+P increased the risk of breast cancer, and the risk elevation persisted, even 3 years after treatment had stopped [Heiss G et al. JAMA 2008]. In contrast, E-alone reduced the risk of breast cancer; data that were collected over a follow-up of nearly 11 years showed a significantly lower cumulative breast cancer incidence that was associated with E-alone compared with placebo (26% vs 34%; HR, 0.75; 95% CI, 0.51 to 1.09) [LaCroix AZ et al. JAMA 2011]. Whether these findings are specific to conjugated estrogen or also apply to estradiol and other estrogen-alone formulations remains unknown. The effects of hormone therapy on breast cancer risks were consistent across age groups, thus showing a minimal modifying effect of age.
Evidence-Based Decision-Making for Hormone Therapy
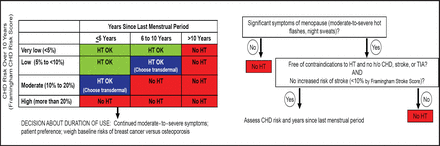
Dr. Manson reiterated that postmenopausal hormone therapy should not be prescribed for the express purpose of preventing chronic disease. However, she added, “In a recently menopausal woman with moderate-to-severe menopausal symptoms, concern about CHD risk from hormone therapy should not be a major factor in decision-making.” She presented a decision-making flowchart to help clinicians choose appropriate candidates for hormone therapy (Figure 2). A woman's cardiac risk should also be considered before starting therapy, especially given that more favorable lipid status and low baseline risk of CHD have been associated with a reduced risk of cardiovascular events with hormone therapy [Bray PF et al. Am J Cardiol 2008]. In some situations, transdermal estrogen may be a better choice than oral estrogen, as the transdermal formulation is less likely to be associated with increased risks of adverse events. In deciding on duration of therapy, Dr. Manson suggested treating only for menopausal symptoms and trying to discontinue treatment within 4 to 5 years; for women with persistent vasomotor symptoms, it will be important to weigh the baseline risks of breast cancer versus osteoporosis.
Hormone Therapy (HT) Decision-Making Flowchart.
CHD=coronary heart disease, TIA=transient ischemic attack [Adapted from Manson J. Harrison's Principles of Internal Medicine 2008]. Reproduced with permission from J. Manson, MD, DrPH.
Questions Remaining
Additional research on hormone therapy in early menopause is needed to answer several remaining questions. Among the ongoing studies is the ELITE trial (NCT00114517), which is designed to examine the effects of oral 17B-estradiol on the progression of subclinical atherosclerosis in healthy postmenopausal women. In addition, the KEEPS trial (NCT00154180) involves coronary imaging to determine the effect of hormone therapy on atherosclerotic progression among younger women who are treated early after menopause, as well as an assessment of cognitive function, quality of life, and mammographic breast density. The data that emerge from these studies will arm clinicians and their patients with more information on the risks and benefits of postmenopausal hormone therapy.
- © 2011 MD Conference Express