Summary
Drug-eluting stents (DES) are commonly used to treat coronary artery disease, because they reduce in-stent thrombosis and the need for repeat revascularization compared with bare-metal stents. However, there are safety concerns regarding the infrequent but life-threatening complication of stent thrombosis. Further development of DES with sustained drug release is hypothesized to represent an even safer alternative.
- Interventional Techniques & Devices
- Coronary Artery Disease Clinical Trials
Drug-eluting stents (DES) are commonly used to treat coronary artery disease (CAD), because they reduce in-stent thrombosis and the need for repeat revascularization compared with bare-metal stents. However, there are safety concerns regarding the infrequent but life-threatening complication of stent thrombosis. Further development of DES with sustained drug release is hypothesized to represent an even safer alternative.
One-year data from the RESOLUTE US trial (NCT00726453), a comparison of a new zotarolimus DES with a hydrophilic biocompatible polymer that provides prolonged drug release (180 days compared with 14 days in the older generation), suggest that the RESOLUTE zotarolimus-eluting stent (R-ZES) is noninferior to historical results of the ENDEAVOR zotarolimus-eluting stent (E-ZES) in rates of clinical restenosis, death, myocardial infarction (MI), and stent thrombosis at 1 year. The results were presented by Martin B. Leon, MD, Columbia University, New York, New York, USA.
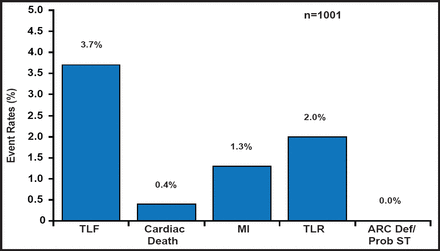
RESOLUTE US was a prospective, observational study that evaluated the clinical effectiveness of the R-ZES in a US population. The study comprised patients (n=1402) with de novo native coronary lesions that were suitable for 1- or 2-vessel treatment with stents from 2.25 to 4.0 mm in diameter. Subjects were enrolled with clinical follow-up only (n=1242) or with angiographic follow-up (n=160). The primary endpoint was 12-month target lesion failure (TLF; defined as a composite of cardiac death, MI, and clinically driven target lesion revascularization [TLR]) compared with historical data from the E-ZES clinical trials. The primary analysis consisted of data from the patients in the clinical cohort who underwent only single lesion revascularization with a 2.5-mm–3.5-mm stent (n=1001). The other 241 patients either had 2 lesions that were treated and/or received a 2.25-mm stent. Completeness of follow-up at 1 year was analyzable in 982 of the 1001 patients.
A total of 1402 subjects were enrolled in this observational cohort. The mean age was 64 years, most were men (68%), one-third were diabetic, the mean target vessel diameter was relatively small at 2.59±0.47 mm, and dual antiplatelet therapy use was 93% at 1 year. At 1 year, TLF occurred in 36 of the 982 patients with complete follow-up, which is a rate of 3.7% versus 6.5% (70/1076) in the E-ZES historical controls (ie, a risk difference of −2.8%, p<0.001 for noninferiority). The development of secondary endpoints was also low (Figure 1). The TLF rate in the overall clinical cohort (n=1402) was 4.7%. The 12-month rate of stent thrombosis was 0.1%, which occurred exclusively in subjects with small-vessel, 2.25-mm stents.
Main Analysis Cohort: 12-Month TLF, Cardiac Death, MI, and TLR.
In summary, RESOLUTE US reported a similar rate of events with the R-ZES next-generation DES compared with earlier E-ZES trials. The low 1-year incidence of in-stent thrombosis and the low need for repeat revascularization that was achieved with very high compliance of dual antiplatelet therapy are reassuring in challenging patients with diabetes mellitus and small-sized vessels. Further follow-up is required to demonstrate long-term efficacy and safety.
- © 2011 MD Conference Express