Summary
Patients with venous disease can be treated with a variety of different treatment strategies and devices.
- thrombotic disorders
- cardiac imaging techniques
- interventional radiology
- thromboembolic disease
- interventional techniques & devices
Patients with venous disease can be treated with a variety of different treatment strategies and devices. Julian J. Javier, MD, Naples Vein Center, Naples, Florida, USA, discussed advances in the field of superficial venous disease. The current treatment of superficial venous disease is based on the surgical removal of the affected vein segment or ablation therapy. Newer nonthermal ablation techniques have emerged including mechanical occlusive chemical ablation, polidocanol, cyanoacrylate glue, and V-block occlusive devices. All of these techniques cause limited pain and have very short or immediate recovery. Long-term data are currently lacking, and as a result, providers may not be reimbursed for these procedures. New thermal ablation techniques have also been developed, including endovenous laser ablation treatment, steam ablation, radiofrequency, and endoluminal tumescent anesthesia delivery.
Raghu Kolluri, MD, Ohio Health System/Riverside Methodist Hospital, Columbus, Ohio, USA, discussed treating venous insufficiency on the basis of a sonographer's report. Through several case studies, Dr. Kolluri illustrated how sonographic venous incompetence studies can aid in determining reflux, or lack thereof, through the veins in the lower extremities. When combined with knowledge of the lower extremity anatomy, one can determine the best vein and region to ablate. He highlighted that deep and superficial veins and perforators should be assessed, as venous insufficiency is not always due to the great saphenous vein or the small saphenous vein.
Jeffrey G. Carr, MD, Vein Center of East Texas, Tyler, Texas, USA, discussed the use of laser and radiofrequency venous ablation. The first step is to identify the best access site, which can vary on the basis of anatomy and therapeutic goals. The use of tumescent anesthesia not only creates a painless procedure but also functions as a heat sink to draw the thermal energy to the vein wall and acts to protect surrounding tissues from the thermal energy. He noted that the tumescent fluid should be delivered to completely surround and halo around the superficial veins. It should surround the vein in order to empty the blood and cause direct contact between the catheter and the vein wall. The catheter tip should be placed ≥ 2 cm from the saphenofemoral junction and superficial epigastric vein. Dr. Carr discussed various techniques to perform thermal ablation in variant anatomy and perforators. He noted that 90% of venous ulcers are associated with incompetent perforators. He concluded that knowledge and experience with thermal ablation techniques for chronic venous insufficiency can lead to highly successful outcomes for patients with impaired quality of life, as well as advanced limb-threatening, nonhealing wounds and ulcers.
Gregory J. Mishkel, MD, Prairie Heart Institute, Springfield, Illinois, USA, discussed the rationale for aggressive therapy for iliofemoral deep vein thrombosis (DVT). Commonly used treatments for iliofemoral DVT, such as compression stockings, do not effectively prevent postthrombotic syndrome (PTS) [Kahn SR et al. Lancet 2014]. However, multiple studies have demonstrated that manual or medical clot removal does prevent PTS (Table 1). For example, the Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion trial [TORPEDO; Sharifi M et al. Catheter Cardiovasc Interv 2010] demonstrated that percutaneous endovenous intervention (PEVI) plus anticoagulation is superior to anticoagulation alone for decreasing the risk for venous thromboembolism (VTE) and PTS in patients with symptomatic DVT. The randomized Catheter-Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis study [Enden T et al. Lancet 2012] found that treatment of iliofemoral DVT with catheter-directed thrombolysis (CDT) resulted in a lower rate of PTS at 24 months and a higher rate of iliofemoral patency at 6 months compared with standard treatment (p = .047 and p = .012, respectively). Guideline recommendations by the American Heart Association suggest that CDT or pharmacomechanical catheter-directed thrombectomy should be considered in patients who experience thrombus extension or clinical deterioration and have a low bleeding risk [Jaff MR et al. Circulation 2011]. Patients who have had DVT symptoms for > 21 days should not receive systemic thrombolysis or CDT or pharmacomechanical CDT. In addition, greater thrombus removal results in a lower rate of PTS. A blinded study of patients with iliofemoral DVT who received treatment with CDT demonstrated that there was a significant association between PTS score and thrombus clearance [Comerota AJ et al. J Vasc Surg 2012]. Multiple devices are available to perform passive diffusion of lytic therapy, mechanical thrombectomy, or lytic assisted devices. Mechanical thrombectomy is performed by suction devices or assisted suction devices. Lytic assisted devices include pharmacomechanical CDT devices that directly deliver pharmacotherapy in addition to mechanical thrombectomy if warranted, and ultrasound-based systems.
Prevention of PTS by Clot Removal
Robert M. Schainfeld, DO, Massachusetts General Hospital, Boston, Massachusetts, USA, discussed how inferior vena cava (IVC) reconstruction differs from iliofemoral intervention. Using a case study, he described steps involved in diagnosing and treating a chronic IVC obstruction using a combination of endovascular techniques including stenting in successfully revascularizing a thrombosed IVC. Endovenous treatment of IVC obstruction has a technical success rate of 100% for stenoses and 66% for occlusions, with a primary patency of 58%, although a primary-assisted patency rate of 82% at 3 years [Raju S et al. J Vasc Surg 2006]. About 74% of patients were pain free, and 51% experienced resolution of edema at 3.5 years. However, thrombosis can occur in the context of an IVC filter, which can be successfully treated by stenting [Neglén P et al. J Vasc Surg 2011]. A stent placed below the filter, rather than across, resulted in an improved patency rate.
Dr. Schainfeld also discussed complications that are associated with IVC filters. IVC filters are indicated only when anticoagulation is contraindicated, when venous thromboembolic events recur despite therapeutic anticoagulation or if a significant complication of anticoagulation is incurred. Importantly, the US Food and Drug Administration (FDA) issued a warning regarding adverse events of IVC filters in 2010 that include device migration, embolization, perforation, and filter fracture [http://www.fda.gov/safety/ucm221707.htm Accessed June 16, 2014]. Therefore, the FDA recommends that filters be removed when prevention of pulmonary embolism (PE) is no longer required. In a retrospective spanning 26 years involving > 1700 patients, PE occurred after filter placement in 5.6%, and IVC thrombosis occurred in 2.7% [Athanasoulis CA et al. Radiology 2000]. A study of different types of filters found varying rates of IVC thrombosis or occlusion [Stein PD et al. Am J Cardiol 2004].
Mohsen Sharifi, MD, Arizona Cardiovascular Consultants and Vein Clinic, Mesa, Arizona, USA, described his current practice of anticoagulant and antiplatelet therapy after acute and chronic DVT intervention. In patients who have had prior thrombus removal by any method, Dr. Sharifi recommended parenteral anticoagulation, oral anticoagulation, a thrombolytic agent, and an antiplatelet agent. To prevent recurrence of thromboembolism, a meta-analysis of the Aspirin to Prevent Recurrent Venous Thromboembolism [Brighton TA et al. N Engl J Med 2012] and Warfarin and Aspirin [Beccattini C et al. N Engl J Med 2012] trials demonstrated that aspirin is favorable compared with placebo in VTE recurrence and major vascular events; however, it is associated with a greater risk for clinically relevant bleeding. In the TORPEDO trial [Sharifi M et al. J Endovasc Ther 2012], patients were randomly assigned to undergo PEVI or standard therapy; all patients in the PEVI arm received uninterrupted anticoagulation and an acetylsalicylic acid for ≥ 6 months. Total VTE and PTS occurred less frequently in the PEVI arm compared with the control group at both 6 and 30 months.
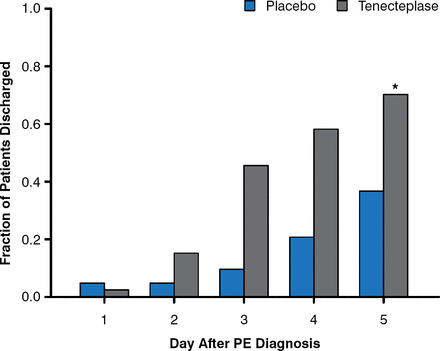
Ido Weinberg, MD, MSc, Massachusetts General Hospital, Boston, Massachusetts, USA, discussed the rationale behind the aggressive management of submassive PE. Following submassive PE, right ventricular dysfunction that results in higher right ventricular systolic (RVSP) can occur [Kline JA et al. Chest 2009]; however, patients who received recombinant tissue plasminogen activator experienced a decrease in Doppler-derived RSVP at 6 months compared with diagnosis. In addition, thrombolysis of submassive PE resulted in less treatment escalation [Konstantinides S et al. N Engl J Med 2002] and a lower rate of death or hemodynamic decompensation as a result of decreased hemodynamic collapse [Meyer G et al. N Engl J Med 2014]. However, thrombolytic therapy carries an increased risk for moderate to severe bleeding and stroke compared with placebo. The Tenecteplase or Placebo: Cardiopulmonary Outcomes at Three Months trial [Kline JA et al. J Thromb Haemost 2014] demonstrated that patients with PE who received tenecteplase require fewer days of care by the intensive care unit and were discharged more quickly compared with patients who received placebo (Figure 1). The Ultrasound Accelerated Thrombolysis of Pulmonary Embolism trial [Kucher N et al. Circulation 2014] demonstrated that right ventricular dysfunction resolved more frequently in patients who underwent ultrasound-assisted CDT compared with those who only received heparin at 24 hours and 90 days (p = .01 and p = .003, respectively), with no reports of major bleeding. Similarly, the Prospective, Single-Arm, Multi-Center Trial of EkoSonic® Endovascular System and Activase for Treatment of Acute Pulmonary Embolism trial [NCT01513759; Piazza G et al. ACC 2014 (abstract 407-04)] demonstrated that mean pulmonary artery systolic pressure decreased incrementally from preprocedure to postprocedure to 48 hours postprocedure with ultrasound-facilitated catheter-directed low-dose fibrinolysis in patients with acute massive and submassive PE. Major bleeding was reported in 11% of patients, but there were no cases of intracranial hemorrhage.
Hospital Discharge Rate Following PE Diagnosis
PE=pulmonary embolism. *p = .002, Chi Square.
Reproduced from Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12:459–468. With permission from John Wiley and Sons.
Dr. Mishkel also discussed the case of deep venous intervention that he most regretted. The patient was a 61-year-old man with morbid obesity and multiple comorbidities who had a history of DVT and saddle PE. The patient received chronic warfarin therapy, and a hypercoagulation panel identified high levels of factor VIII. After receiving a Wallstent that was larger than needed because of the inventory on hand, the patient experienced multiple occlusions, including 1 within the stent, and required multiple procedures to resolve the occlusions. Dr. Mishkel stated that he wished he had never taken on the case because the patient had a borderline indication, the patient's need for warfarin was unclear, and the stent and balloon were inappropriately sized. He noted that this case illustrates the importance of choosing appropriate patients and an appropriate device.
- © 2014 MD Conference Express®