Summary
This article provides a broad overview of the methodology, major findings, and impact of the 3 major transcatheter aortic valve replacement (TAVR) trials (PARTNER, CoreValve, and CHOICE) along with some perspective.
- valvular disease
- interventional techniques & devices
- cardiology clinical trials
Martin B. Leon, MD, Columbia University Medical Center, New York, New York, USA, provided a broad overview of the methodology, major findings, and impact of the 3 major transcatheter aortic valve replacement (TAVR) trials (PARTNER, CoreValve, and CHOICE) along with some perspective.
PARTNER
The PARTNER I trials (1A, 1B, and continued access registry) [Kodali SK et al. N Engl J Med 2012; Makkar RR et al. N Engl J Med 2012; Smith CR et al. N Engl J Med 2011; Leon MB et al. N Engl J Med 2010] comprised a total of 3128 patients with symptomatic severe (high-risk and inoperable) aortic stenosis (AS) who were treated with either TAVR using the SAPIEN Transcatheter Heart Valve device or standard aortic valve replacement (AVR) surgery. In high-risk patients, comparisons were also made between transfemoral and transapical accesses. When comparing standard AVR and TAVR in inoperable patients, all-cause mortality was significantly higher for AVR patients (HR, 0.53; 95% CI, 0.41 to 0.68; p < .0001). The number needed to treat at 3 years was 3.7 patients. In high-risk patients, all-cause mortality at 3 years was similar between the 2 groups.
Predictors of mortality included strokes, major vascular complications, and major bleeding. After 3 years, the 2 treatments were similar with respect to mortality, reduction in symptoms, and improved valve hemodynamics, but paravalvular regurgitation was more frequent after TAVR and was associated with increased late mortality.
Other findings from the PARTNER trial include improved clinical outcomes in transfemoral versus transapical TAVR patients with marked improvement in all quality-of-life metrics. Earlier recovery in NYHA class compared with AVR was reported in the transfemoral cohort. Subanalyses have indicated that compared with surgery, clinical outcomes are better in women, patients with diabetes, those with significant mitral regurgitation, and cases of patient–prosthesis mismatch.
The ongoing PARTNER II trial [NCT01314313] is similar in design to PARTNER I but larger (n = 3716). It includes patients with moderate disease and replaces the original SAPIEN device with the SAPIEN XT, a lower profile device that is currently being used in Europe.
The PARTNER trials established TAVR as the standard of care for inoperable patients and as an acceptable/preferred therapy in high-risk patients. Strokes and paravalvular regurgitation are of concern following TAVR.
CoreValve
The CoreValve Platform is a multilevel self-expanding valve designed to maintain coronary access and mitigate paravalvular aortic regurgitation. In the United States, the safety and efficacy of the CoreValve have been evaluated in 2 pivotal trials: a registry in extreme-risk patients and a randomized controlled trial in patients with severe AS at high surgical risk.
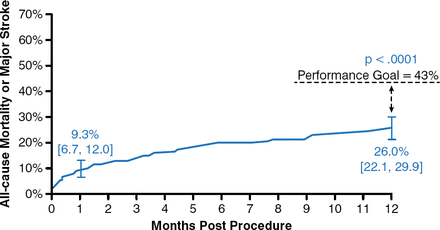
In the extreme-risk study (n = 489), the primary end point of all-cause mortality or major stroke at 12 months was 26.0% (95% CI, 21.6 to 29.9) versus the 43.0% prespecified objective performance goal (p < .0001; Figure 1) [Popma JJ et al. J Am Coll Cardiol 2014]. Procedural events at 30 days included life-threatening or disabling bleeding (12.7%), major vascular complications (8.2%), and need for permanent pacemaker placement (21.6%). Paravalvular aortic regurgitation was lower with the CoreValve device at 12 months compared with discharge.
CoreValve Improved All-Cause Mortality or Major Stroke Outcome
Reproduced from Popma JJ et al. Transcatheter Aortic Valve Replacement Using a Self-Expanding Bioprosthesis in Patients With Severe Aortic Stenosis at Extreme Risk for Surgery. J Am Coll Cardiol. 2014;63(19)1972–1981. With permission from Elsevier.
In the high-risk study, patients were randomly assigned to TAVR (n = 390) with the self-expanding transcatheter valve or to surgical AVR (n = 357) [Adams DH et al. N Engl J Med 2014]. The primary end point was the rate of death from any cause at 1 year evaluated with the use of both noninferiority and superiority testing. After 1 year, the rate of death from any cause was significantly lower (p = .04 for superiority) in the TAVR group than in the surgical group (14.2% vs 19.1%). Major stroke rates were 7.0% for AVR and 5.8% for TAVR (not significant).
CoreValve performed well in both high- and extreme-risk patients with acceptable risk of stroke and vascular complications. This is the first TAVR study to demonstrate clear incremental benefits (all-cause mortality at 1 year and valve hemodynamics) compared with AVR.
CHOICE
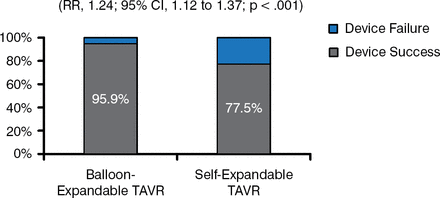
The CHOICE randomized clinical trial compared transcatheter deployment of aortic valves using either a balloon-expandable (SAPIEN XT; n = 121) or self-expandable (CoreValve; n = 120) system [Abdel-Wahab M et al. JAMA 2014]. The study was also performed in patients with severe AS at high surgical risk. The primary end point was device success (successful vascular access and deployment of the device and retrieval of the delivery system, correct position of the device, and intended performance of the heart valve without moderate or severe regurgitation).
Device success occurred in 95.9% of patients in the balloon-expandable valve group and 77.5% patients in the self-expandable valve group (p < .001; Figure 2), which the investigators attributed to the need for more multiple-valve implantations and a higher frequency of paravalvular regurgitation in the self-expanding group.
Device Success With Balloon-Expandable TAVR Better Than With Self-Expandable TAVR
RR=relative risk; TAVR=transcatheter aortic valve replacement.
Reproduced from Popma JJ et al. Transcatheter Aortic Valve Replacement Using a Self-Expanding Bioprosthesis in Patients With Severe Aortic Stenosis at Extreme Risk for Surgery. J Am Coll Cardiol. 2014;63(19)1972–1981. With permission from Elsevier.
Major clinical outcomes (death, stroke, and myocardial infarction vascular complications) at 30 days were similar between the 2 groups. There was improved valve hemodynamics but increased paravalvular aortic regurgitation with the self-expandable system. This was a small and somewhat controversial study with a questionable primary end point that may exaggerate the clinically meaningful difference between the 2 systems.
Conclusions
TAVR has become the preferred therapy for high-risk AS patients, transcending the usual bounds of a new interventional therapy while transforming the pathways for managing patients with complex cardiovascular disease.
- © 2014 MD Conference Express®