Summary
Results from ABSORB II [Serruys PW et al. Lancet. 2014], the first study to compare an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent, demonstrated similar 1-year clinical outcomes in patients with coronary artery disease.
- Coronary Artery Disease
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Coronary Artery Disease
- Cardiology Clinical Trials
- Cardiology
- Interventional Techniques & Devices
Results from ABSORB II [Serruys PW et al. Lancet. 2014], the first study to compare an everolimus-eluting bioresorbable scaffold with an everolimus-eluting metallic stent, demonstrated similar 1-year clinical outcomes in patients with coronary artery disease. Data were presented by Patrick W. Serruys, MD, Imperial College, London, United Kingdom.
ABSORB II is an ongoing, randomized, single-blind, multicenter clinical investigation comparing clinical and procedural outcomes between the ABSORB everolimus-eluting bioresorbable vascular scaffold system and the everolimus-eluting coronary stent (XIENCE). The coprimary end points are vasomotion (change in mean lumen diameter before and after nitrate administration at 3 years) and the difference between minimum lumen diameter (after nitrate administration) after the index procedure and at 3 years. Prof Serruys presented the secondary clinical and procedural outcomes; a composite clinical end point of death, myocardial infarction (MI), and coronary revascularization; device and procedural success; and angina status.
ABSORB-II included patients (n = 501) aged 18 to 85 years with evidence of myocardial ischemia and up to 2 de novo native lesions in different epicardial vessels randomized to either the ABSORB scaffold (n = 335) or XIENCE stent (n = 166). Procedural performance was assessed by quantitative angiography and intravascular ultrasound (IVUS). Device and procedural success were presented in percentage. Angina status was assessed by the Seattle Angina Questionnaire (SAQ). Exercise testing occurred at 6 and 12 months. Post hoc adverse event (AE) reporting was used to determine cumulative angina rate.
Approximately 84% of patients had single vessel disease, of which the majority (98%) was class B1/B2 lesions. There were no differences in procedural details per lesion except for nominal diameter of last balloon used (ABS ORB 3.08 mm vs XIENCE 3.16 mm; P = .02) and maximum last balloon pressure used (ABSORB 14.23 atm vs XIENCE 15.03 atm; P = .01).
Clinical device and procedural success rates for both devices were > 95%. There was no difference in the cumulative incidence of the composite clinical outcome of death, MI, or revascularization (7% and 9% ABSORB and XIENCE arms, respectively; P = .47). Acute lumen gain whether by angiography (ABSORB 1.15 mm vs XIENCE 1.46 mm) or IVUS (Absorb 2.85 mm2, XIENCE 3.60 mm2) was significantly (both P < .001) lower in the ABSORB arm compared with the XIENCE arm. The investigators suggested this may be attributable to the greater pressure and larger size of balloon used during the postimplantation dilatation with XIENCE.
One definite acute, 1 definite subacute, and 1 probable late incidence of scaffold thrombosis was documented in the ABSORB arm and none in the XIENCE arm. The per-protocol periprocedural MI rates were 4% and 1% in the ABSORB and XIENCE arms (P = .16), respectively. There were 17 (5%) major cardiac AEs with ABSORB compared with 5 (3%) events in the XIENCE arm. The most common AEs were MI and target-lesion revascularization. Myocardial biomarkers (troponin, creatine kinase, creatine kinase-MB) did not indicate a substantial difference in myonecrosis between the 2 devices.
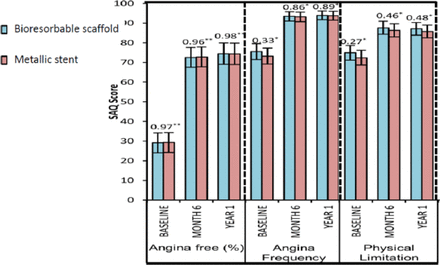
Exercise performance and angina status as assessed by SAQ were comparable (Figure 1). Cumulative rates of new, recurrent, or worsening angina were lower in the ABSORB (21.8%) group compared with the XIENCE (30.5%) group (P = .04). If the first 7 days including hospitalization were excluded, the rates were 16.4% and 25.6% (P = .02). This post hoc, hypothesis-generating observation warrants further physiological and clinical investigation.
SAQ Exercise Performance and Angina Status
*P value from post hoc t test; **†P value from post hoc χ2 test.
SAQ, Seattle Angina Questionnaire.
Reproduced from Serruys PW et al. A bioresorbable everolimus-eluting scaffold versus a metallic everolimus-eluting stent for ischaemic heart disease caused by de-novo native coronary artery lesions (ABSORB II): an interim 1-year analysis of clinical and procedural secondary outcomes from a randomized controlled trial. Lancet. 14 Sept 2014; In Press, Corrected Proof. Copyright 2014, with permission from Elsevier.
- © 2014 MD Conference Express®