Summary
Interventional cardiology had its beginnings in Andreas Gruentzig's (1939–1985) dream to “accomplish catheter-based percutaneous treatment of vascular disease in alert, awake patients” [Sievert H et al., eds. Percutaneous Interventions for Congenital Heart Disease. USA: CRC Press; 2007:29]. This article provides an overview of the advances in interventional cardiology that have occurred since Prof. Gruentzig performed the first balloon angioplasty in 1977 using a self-made catheter.
- interventional techniques & devices
Interventional cardiology had its beginnings in Andreas Gruentzig's (1939–1985) dream to “accomplish catheter-based percutaneous treatment of vascular disease in alert, awake patients” [Sievert H et al., eds. Percutaneous Interventions for Congenital Heart Disease. USA: CRC Press; 2007:29]. Martin B. Leon, MD, Columbia University Medical Center, New York, New York, USA, provided an overview of the advances in interventional cardiology that have occurred since Prof. Gruentzig performed the first balloon angioplasty in 1977 using a self-made catheter.
Although a major advancement at the time, balloon angioplasty was soon found to be associated with frequent dissections, recoil, and poor angiographic outcomes. It was ineffective in some types of lesions (eg, calcifications), required surgical backup due to the risk of acute vessel closure, and was frequently associated with restenosis.
From 1988 to 1993, interventional cardiology's “new device” era was represented by the introduction of laser angioplasty, atherectomy, and stents. Again, despite much progress, including some improvement in the treatment of complex lesions, this new era was marked by concerns in the form of frequent complications, the need for greater/more costly operator expertise, and higher rates of restenosis. One device—the Palmaz-Schatz stent—stood out, however, for its ability to increase the safety, predictability, and definitiveness of angioplasty.
Between 1993 and 1998, the volume of coronary procedures grew. A new era of evidence-based medicine (EBM) was ushered in with the US Food and Drug Administration's 1994 approval of the Palmaz-Schatz stent based on two randomized trials, BENESTENT and STRESS. Stenting became the mainstream with the 1995 publication of a study by Colombo and colleagues showing reduced bleeding complications when the Palmaz-Schatz stent was inserted using intravascular ultrasound-guided high-pressure postdilation followed by antiplatelet therapy using only aspirin plus ticlopidine [Colombo A et al. Circulation 1995].
The years between 1998 and 2008 witnessed the birth of more new technology and a true subspecialty. Bare-metal stents (BMS) evolved to drug-eluting stents (DES) but, while restenosis became less of a concern, a new problem—late stent thrombosis—emerged. EBM flourished during these years and there was an expansion of the technology associated with extra-coronary targets.
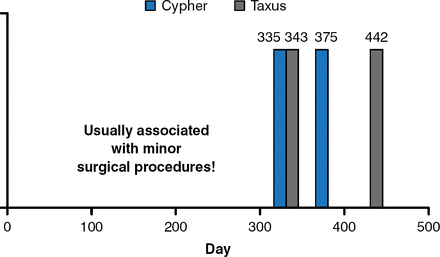
The first human trial of a DES was conducted in Brazil in 1999, and it was a transformative technology. However, in 2004, 5 years after the first use of DES, reports began to appear regarding the emergence of late stent thrombosis (LST) after discontinuation of antiplatelet therapy. The first report was in regards to 4 patients following implantation of either the Taxus or Cypher stents. All cases arose soon after anti-platelet therapy was interrupted and resulted in myocardial infarction (MI; Figure 1) [McFadden EP et al. Lancet 2004]. These events were confirmed in other centers [Wenaweser P et al. J Am Coll Cardiol 2008; Mauri L et al. N Engl J Med 2007]. Thus, although late-loss was near zero and restenosis was very low, DESs had their own set of problems, which included delayed healing, hypersensitivity, inflammation, abnormal vasomotion, incomplete apposition and, most importantly, the unpredictable and extremely problematic phenomenon of LST.
Late DES Thrombosis After Discontinuation of Antiplatelet Therapy
Reproduced with permission from M Leon, MD. Source: McFadden EP et al. Lancet 2004.
Following these reports and the ensuing firestorm at the 2006 meeting of the European Society of Cardiology, the use of DESs in the United States decreased from 89.3% to 60.9% over about a 2-year period. In an effort to better define the risk of stent thrombosis with DES, Kirtane and colleagues [Circulation 2009] published a meta-analysis, which included data from 9470 patients in 22 randomized controlled trials and 182,901 patients in 34 observational studies, and found no significant differences in the long-term rates of death or MI after the use DES or BMS for either off-label or on-label indications (Table 1).
DES Treatment Effect Estimates
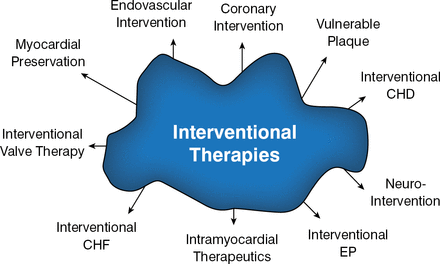
During this period, the evolution of interventional cardiology was marked by an increased reliance on the use of EBM (> 2500 peer-reviewed, DES-focused manuscripts were published between 2002 and 2012) and the development of the second-generation DESs. There was also an explosion in catheter-based therapies for other cardiovascular conditions (Figure 2).
Potential Uses of Interventional Cardiology in 2001
CHD=congenital heart disease; CHF=congestive heart failure; EP=electrophysiology.
Reproduced with permission from M Leon, MD.
The most recent phase of the evolution of interventional cardiology (2008 to 2013) was characterized by the use of extravascular and mainstream therapies (such as for valvular heart disease, hypertension, and atrial fibrillation). This latest change is marked by the rise in transcatheter aortic valve replacements (TAVR) and the formation of multidisciplinary heart teams. Today, EBM is mandated and international networks for innovation, clinical research, and education have been developed.
Looking to the future, lessons learned over time within the field of interventional cardiology needed to be applied in other ways. As at 2014, TAVR devices such as the SAPIEN Transcatheter HeartValve and CoreValve have been used to treat > 100,000 patients in > 750 interventional centers around the world. Multidisciplinary collaboration in the form of heart teams has become common, multimodality imaging the rule, and more facilities are developing dedicated catheterization laboratories and operating rooms.
The focus on EBM continues. Four PARTNER manuscripts were published in the New England Journal of Medicine between October 2010 and May 2012 [Leon MB et al. 2010; Smith CR et al. 2011; Makkar RR et al. 2012; Kodali SK et al. 2012], and the mortality reduction shown in PARTNER 1 led to TAVR becoming the standard of care for patients with aortic stenosis who are not suitable for surgery. Important new studies, such as the Medtronic CoreValve US Pivotal Trial [Adams DH et al. N Engl J Med 2014], indicate that TAVR may be better than surgery in some patients. Dr. Leon believes that in 5 to 10 years, most patients with severe aortic stenosis requiring surgery will be treated with TAVR (even low-risk patients).
Research shows that new market segments, such as TAVR, hypertension, left atrial appendage occlusion, and transcatheter mitral valve replacement, will likely exceed the percutaneous coronary intervention (PCI) market size by 2020. A major current effort is to redirect intravascular interventional therapies to address “mainstream” cardiovascular and noncardiovascular disease. Such a transition requires that interventionalists become integrated members of a multidisciplinary team, learn new cognitive skills, and transform from isolated proceduralists back to their roots as engaged therapists.
Between 1975 and 2005, there was an 80% reduction in mortality from heart attack, which was achieved primarily through technological innovation. Interventional cardiologists are facing challenges that have broken the cycle of innovation (Table 2) through which new technology is brought to patients.
Challenges to Innovation
As for the future, Dr. Leon stated that there is a need to start fixing the pump instead of the valves and vessels. He sees the next big breakthrough area as being in new heart failure therapies. These will include sensors to monitor therapy, left-ventricular remodeling devices, contractility modulation-micro-ventricular-assist devices, interatrial shunt implants, and stem-cell therapies. Improvements in the treatment of coronary artery disease are also needed. For this, new technologies include bioabsorbable stents, drug-coated balloons, advanced balloon technologies, vascular remodeling, and new therapeutic targets. Other areas of interest are shown in Table 3.
Interventional Perspectives: Future Directions
Dr. Leon believes there has never been a better time to be an interventional cardiologist. His message is to adapt and evolve but remember that it is still all about the patient.
- © 2014 MD Conference Express®