Summary
Transcatheter aortic valve replacement (TAVR) has become a treatment option for patients with severe aortic stenosis who either are not surgical candidates or are at high risk for surgical complications. The results from the US CoreValve pivotal trials support the safety and efficacy of the CoreValve prosthesis in these patient populations. This article discusses detailed data from the CoreValve trials
- Valvular Disease
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Valvular Disease
- Cardiology Clinical Trials
- Interventional Techniques & Devices
- Cardiology
Transcatheter aortic valve replacement (TAVR) has become a treatment option for patients with severe aortic stenosis who either are not surgical candidates or are at high risk for surgical complications. The results from the US CoreValve pivotal trials support the safety and efficacy of the CoreValve prosthesis in these patient populations. In high- and extreme-risk patients with aortic stenosis, TAVR, using a self-expanding transcatheter aortic valve bioprosthesis, results in significantly higher rates of 1-year survival compared with surgical aortic valve replacement (SAVR). TAVR performed better than a prespecified objective performance goal (OPG) set prior to the study [Adams DH et al. N Engl J Med 2014; Popma JJ et al. J Am Coll Cardiol 2014]. One-year outcomes from the CoreValve ADVANCE study confirmed these findings [Linke A et al. Eur Heart J 2014].
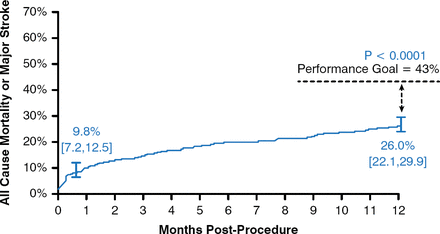
Juan Gaspar, MD, Director of Training and Medical Education of Medtronic Cardiovascular in Latin America, presented detailed data from the CoreValve trials. In the extreme-risk trial, 489 patients who were not candidates for SAVR underwent TAVR. This cohort was elderly (mean age, 83.2 ± 8.7 years) and had a high predicted surgical risk on the basis of the Society of Thoracic Surgeons risk score (mean predicted mortality, 10.3 ± 5.5%), and a high proportion had New York Heart Association (NYHA) functional class III or IV heart failure (91.8%). These patients also had high scores on tests for frailty and disability. At 1 year, the rate of all-cause mortality or major stroke was 9.8% at 1 month and 26 in patients treated with TAVR. The results at 1 year were superior to the prespecified OPG of 43% (p < .0001; Figure 1); thus, the trial was considered to have met its primary end point.
All-Cause Mortality or Major Stroke Following TAVR in Extreme-Risk Patients
TAVR=transcatheter aortic valve replacement.
Reproduced from Popma JJ et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972–1981. With permission from Elsevier.
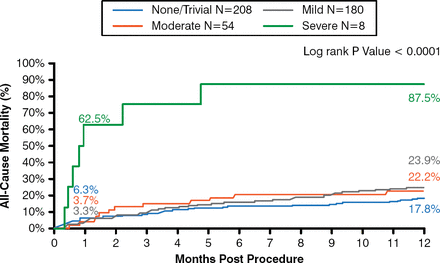
All-cause and cardiovascular mortality were 24.3% and 18.3%, respectively, at 1 year. There were low rates of major stroke at 1 month (2.3%) and 1 year (4.3%). Life-threatening or disabling bleeding was 17.6%, and major bleeding was 28.5% at 1 year. Echocardiographic findings showed mean gradients of 9.55 mm Hg at discharge and 8.86 mm Hg at 1 year, and a more than doubling in orifice size. Severe cases of paravalvular regurgitation (PVR) were infrequent (1.6% at discharge and 0% at 1 year). There was no association between mild or moderate paravalvular leak and late mortality (Figure 2).
No Impact of Paravalvular Leak on Late Mortality Following TAVR
TAVR=transcatheter aortic valve replacement.
Reproduced from Popma JJ et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972–1981. With permission from Elsevier.
The results from the US CoreValve study in extreme risk patients support the safety and efficacy of TAVR therapy in patients deemed not candidates for SAVR.
The second CoreValve US pivotal trial compared TAVR and SAVR in symptomatic patients with severe aortic stenosis. This trial included patients who were candidates for surgery but were at increased risk. Baseline characteristics, including mean age, predicated mortality, NYHA class, history of prior stroke, and rates of prior coronary artery bypass surgery were similar between the operative and nonoperative groups.
The trial tested the noninferiority of TAVR compared with SAVR, and the predefined noninferiority margin was met (p value for noninferiority < .001). TAVR (14.2%) reduced mortality at 1 year compared with SAVR (19.1%, p = .04). The rate of stroke (including major stroke) was not significantly different between TAVR and SAVR at 1 month or at 1 year. Patients treated with TAVR had lower rates of all-cause mortality or major stroke at 1 month (5.9% vs 6.7%) and 1 year (16.3% vs 22.5%).
Patients treated with TAVR had lower rates of major vascular complications, pacemaker implantations, life-threatening bleeding, new-onset or worsening atrial fibrillation, and acute kidney injury compared with those receiving SAVR; however, patients treated with SAVR had significantly lower PVR. By 1 year, 76.2% TAVR patients with moderate or severe PVR at the time of hospital discharge had improved PVR.
Both CoreValve studies showed stable valve function over 1 year, low rates of major stroke, and low rates of moderate or severe aortic regurgitation. Dr. Gaspar concluded that the results from the US CoreValve trials support the use of the TAVR with the self-expanding transcatheter valve in patients with aortic stenosis who are not candidates for SAVR and those patients at high risk for surgical complications.
The editors would like to thank the many members of the 2014 Caribbean Cardiac Society presenting faculty who generously gave their time to ensure the accuracy and quality of the articles in this publication.
- © 2014 MD Conference Express®