Summary
A patent foramen ovale (PFO) is common in the general population and has been associated with cryptogenic stroke, stroke following orthopedic or neurosurgery, migraines with aura, and sleep apnea, as well as other less common conditions (ie, orthodexia, decompression illness, and altitude sickness). This article examines studies on PFO closures, complications with these devices, and left atrial appendage procedures.
- interventional techniques & devices
- cardiology genomics
A patent foramen ovale (PFO) is common in the general population and has been associated with cryptogenic stroke, stroke following orthopedic or neurosurgery, migraines with aura, and sleep apnea, as well as other less common conditions (ie, orthodexia, decompression illness, and altitude sickness). Jonathan M. Tobis, MD, David Geffen School of Medicine, UCLA, Los Angeles, California, USA, discussed some of the studies that have examined PFO closure in these patients.
It remains controversial whether PFO closure has benefit over medical management of the prevention of recurrent stroke. The Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care trial [RESPECT; Carroll JD et al. N Engl J Med 2013] compared the Amplatzer PFO closure device (St. Jude's Medical) with medical management with antiplatelet or warfarin therapy in patients with a PFO and prior cryptogenic stroke. Although the primary intention-to-treat (ITT) analysis showed no significant additional benefit associated with PFO closure (HR, 0.49; 95% CI, 0.22 to 1.11; log-rank p = .08), closure was superior to medical therapy alone in the prespecified per-protocol and as-treated analyses. The overall frequency of serious adverse events did not differ significantly between the two groups.
The Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Stroke (PC) trial also compared the Amplatzer device with medical therapy alone. Over 4 years, closure of a patent foramen ovale for secondary stroke prevention did not result in a significant reduction in the risk of recurrent embolic events or death compared with medical therapy [Meier B et al. N Engl J Med 2013].
When these two studies were combined in a meta-analysis, the difference in favor of the device was significant (p = .02), even in the ITT analysis [Tobis J. Clev Clin J Med 2014].
PFO is also present in ∼50% of individuals with migraine and aura and 50% of individuals with cryptogenic stroke also have migraines [Tobis JM, Azarbal B. Tex Heart Inst J 2005]. Data suggest that people who have migraines with or without aura are at a higher risk of ischemic stroke than is the general population (2.3 and 1.8 times greater risk, respectively [Etminan T et al. BMJ 2005], and almost all are due to the presence of a PFO [Wilmshurt P et al. Am J Cardiol 2006]. The Premium Migraine Trial [NCT00355056] is currently evaluating the impact of PFO closure on the incidence of disabling migraine headaches (ie, 6 to 14 days per month). Results are expected during 2014. Studies have also shown a higher frequency of PFO in patients with obstructive sleep apnea [Beelke M et al. Sleep Med 2003], but randomized trials are needed to assess whether closure is beneficial in these patients.
Dr. Tobis recommends that the cardiologist and neurologist cooperate in the management of patients with PFO and stroke or migraines and that the cardiologist should assume management responsibility for patients with PFO and other conditions, except those with sleep apnea, who should be managed by a sleep specialist.
In the United States, transcatheter atrial septal defect (ASD) closure in the adult patient is performed using either the Amplatzer Atrial Septal Occluder (ASO) or GORE Helex Septal Occluder HSO). Damien Kenny, MB, MD, Rush University Medical Center, Chicago, Illinois, USA, noted that, while both devices are safe and effective, there is an inconsistency of reporting the frequency of erosions and other complications with these devices, and he cautioned the audience to take note of a basis for any calculation of event rates (actual implant versus sales).
In both the ASO and HSO US pivotal trials, the overall rate of complications was lower with a percutaneous device as compared with surgical ASD closure; however, this did not reach statistical significance in the Helex trial [Jones TK et al. J Am Coll Cardiol 2007; Du ZD et al. J Am Coll Cardiol 2002]. The most common device-related event in both studies was device embolization (0.2% with the ASO; 1.7% with the HSO).
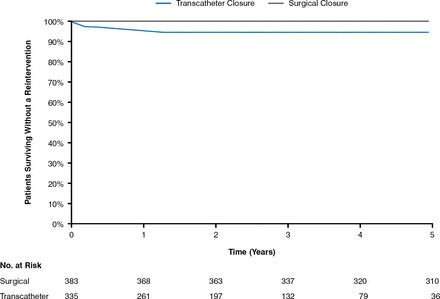
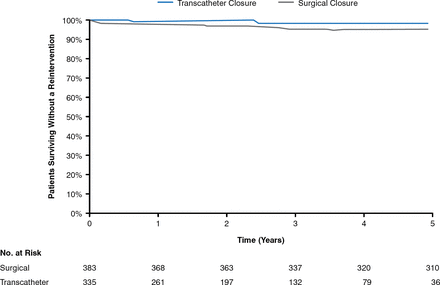
At follow-up, the three most important complications reported are arrhythmia, embolization, and erosion for the ASO and periprocedural pericardial effusions, vessel damage, and thrombus formation for the HSO. Fracture is not an issue with the ASO; however, fracture rates as high as 6.4% have been reported for the HSO [Fagan T et al. Catheter Cardiovasc Interv 2009]. A recent meta-analysis of 28,142 patients (203 studies; 11 different devices) reported periprocedural rates of arrhythmia and heart block with ASD device closure of just under 2.5% and 0.4%, respectively [Abaci A et al. Catheter Cardiovasc Interv 2013]. Rates of thrombosis with both devices are low [Krumsdorf U et al. J Am Coll Cardiol 2004]. A recent efficacy and long-term (5 years) safety study comparing transcatheter versus surgical closure of ASD reported that transcatheter ASD closure was associated with a higher long-term re-intervention rate (7.9% vs 0.3% at 5 years, p = .0038), with a mortality rate similar to surgery (5.3% vs 6.3% at 5 years, p = 1.00; Figures 1 and 2) [Kotowycz MA et al. JACC Cardiovasc Interv 2013].
Reintervention After ASD Closure Kaplan-Meier Estimates for Time to First Reintervention During the First 5 years of Follow-up
ASD=atrial septal defect.
Reproduced from Kotwycz MA et al. Long-Term Outcomes After Surgical Versus Transcatheter Closure of Atrial Septal Defects in Adults. JACC Cardiovasc Interv 2013;6(5):497–503. With permission from Elsevier.
Long-Term Mortality After ASD Closure Kaplan-Meier Estimates for Mortality During the First 5 Years of Follow-up
ASD=atrial septal defect.
Reproduced from Kotwycz MA et al. Long-Term Outcomes After Surgical Versus Transcatheter Closure of Atrial Septal Defects in Adults. JACC Cardiovasc Interv 2013;6(5):497–503. With permission from Elsevier.
Device erosion is an ongoing concern. One study with 28 patients noted higher rates of erosion in patients with deficient aortic rims and with the use of oversized devices [Amin Z et al. Catheter Cardiovasc Interv 2004]. Dr. Kenny noted a high proportion of women (3:1) in this study and suggested that not only were the devices potentially oversized but also that the 26-, 18-, and 34-mm devices appeared overly represented. Of possible concern with these particular devices (as well as the 11-mm device) is the rigidity of the wire mesh.
Dr. Kenny recommends the use of CT if there is a clinical suspicion of “subacute” erosion, more attention to patient sex and device size, and a greater appreciation for how all these parts fit together.
Heart Teams have been used with good success for transcatheter aortic and mitral valve replacement procedures. Mark Reisman, MD, University of Washington, Seattle, Washington, USA, discussed the skills needed for performing left atrial appendage (LAA) closure with the Watchman device.
LAA closure using the Watchman device requires both technical skills and a thorough understanding of the LA anatomy. The fragile structures involved in the procedure, variability in the size and shape of the LAA, the location of the pulmonary veins (PV) in relation to the LAA, and the need to be careful of the pericardium are important aspects when accessing the LA via the transseptal approach. Meticulous attention to sheath management, the evolving role of atrial fibrillation ablation techniques, and the correct us of oral anticoagulants are also part of the needed skill set for performing this procedure.
Potential members of an LAA team include an imaging specialist comfortable with interventions, a partnership/collaboration with a structural heart program (interventional cardiologist [IC] and electrophysiologist [EP]), as well as someone familiar with PV ablation and bailout therapies. Patients should undergo heart contrast computed tomography to determine suitable LAA position, orientation, size, and number of lobes.
A multiphase training program has been created to ensure comprehensive device and procedure training for surgeons. It includes transseptal puncture experience, catheter manipulation in the LA, device delivery and deployment experience, transesophageal echocardiogram and intracardiac echo imaging skills, understanding of anticoagulation, and cardiac intervention complication management skills, including pericardial effusions. During training, practice dynamics are explored, involving an interventional/heart team approach and collaborative EP and IC procedure involvement, assessment for willingness to engage in Therapy Awareness initiatives, ability to draw referrals from regional physicians, and practice dynamics that allow for a cadence of cases to build procedural confidence.
LAA closure using the Watchman device requires a hospital infrastructure that supports Watchman procedures, including highly experienced interventional cardiologists, a multispecialty implanting team, and a dedicated echocardiologist.
- © 2014 MD Conference Express®