Summary
This article presents data from the open-label extension study of the RIDE [A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus; NCT00473382] and RISE [A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus; NCT00473330] trials.
- Diabetes Mellitus
- Ophthalmology Clinical Trials
- Retinal Diseases
- Ophthalmology
- Diabetes Mellitus
- Ophthalmology Clinical Trials
- Retinal Diseases
Charles C. Wykoff, MD, PhD, Retina Consultants of Houston, Houston, Texas, USA, presented data from the open-label extension (OLE) study of the RIDE [A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus; NCT00473382] and RISE [A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus; NCT00473330] trials. The results demonstrated that almost one-fourth of participants in the OLE study maintained vision and retinal anatomy gains achieved during the RIDE and RISE trials without the need for additional ranibizumab injections.
According to Dr Wykoff, upon completion of the core 36-month RIDE and RISE trials, 66% (n = 500) of the 759 originally randomized patients enrolled in the OLE trial. Patients were then examined essentially monthly and treated with 0.5-mg ranibizumab injections on a PRN basis according to predefined retreatment criteria, including evidence of diabetic macular edema (DME) on optical coherence tomography.
There was a wide variation in the frequency of PRN ranibizumab injections during OLE, and 24.2% of patients required no injections (mean = 4.5 injections). Dr Wykoff indicated that the objective of this subanalysis was to further characterize this group of patients who received no injections during OLE and compare them with a group of patients who required many injections during OLE (> 7 annualized injections, or 17.6% of the OLE population).
Analysis of patient baseline and treatment characteristics from RIDE and RISE demonstrated that 10 particular characteristics correlated with ultimate treatment frequency during OLE (Table 1).
Characteristics That Correlated With Treatment Frequency During Open-Label Extension
With respect to baseline characteristics, the duration of clinically significant DME was approximately 10 months shorter in patients receiving no ranibizumab injections during OLE. These patients also had better vision (approximately 4 more letters on the Early Treatment Diabetic Retinopathy Study [ETDRS] chart), better retinal anatomy (approximately 85 μm less mean central foveal thickness [CFT] related to DME), and less severe diabetic retinopathy (DR), including less proliferative DR.
From a treatment perspective, patients who required no ranibizumab injections during OLE required fewer laser applications during the RISE/RIDE trials than those who received many injections during OLE.
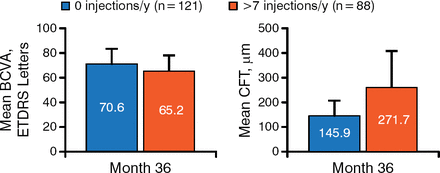
With respect to treatment response during RISE/RIDE, 5 characteristics correlated with ultimate treatment frequency during OLE. Patients who required no ranibizumab injections had better vision (approximately 5 more ETDRS chart letters) and better retinal anatomy (approximately 125 μm less mean CFT related to DME) at month 36 (Figure 1).
Visual Gains and Central Foveal Thickness During Open-Label Extension
BCVA, best corrected visual acuity; CFT, central foveal thickness; ETDRS, Early Treatment Diabetic Retinopathy Study.
Reproduced with permission from CC Wykoff, MD, PhD.
More patients who received no injections during OLE experienced a 2-step greater improvement in DR severity at 36 months (40% vs 19%). These patients also experienced more improvement in the area of dye leakage on fluorescein angiography, with a difference of > 2 mean disc areas of improvement at 36 months compared with patients receiving > 7 injections during OLE. Finally, there was a slight negative correlation between HbA1c levels at 36 months and the need for ranibizumab reinjection during OLE; HbA1c levels slightly increased from baseline to 36 months in patients who required no injections, whereas they remained stable in those who received > 7 injections.
Dr Wykoff noted that although OLE was designed to continue for 2 years, the trial ended after approval of ranibizumab by the US Food and Drug Administration for the treatment of DME. In summary, he noted that during OLE, patients who required no ranibizumab injection were characterized by less severe ocular diabetic disease at baseline and a better response to ranibizumab during the core RIDE and RISE trials.
- © 2014 MD Conference Express®