Summary
Symptoms of cardiotoxicity resulting from cancer therapy, sometimes manifesting years or even decades later, are a problem for survivors. In a special session on cardio-oncology, expert consensus recommendations from the European Association of Cardiovascular Imaging on best practices for detecting signs of cardiotoxicity in cancer survivors were reviewed.
- multimodality imaging
- cardiotoxicity
- radiotherapy
- chemotherapy
- radiation-induced heart disease
- cardiovascular risk
- trastuzumab
- anthracycline
- monoclonal antibody
- tyrosine kinase inhibitor
Cancer Treatments and Cardiac Effects: What Is the Scope of the Problem?
In the first of 4 clinical seminars in a session on multimodality imaging of cardiotoxicity emerging from cancer treatment, Marielle Scherrer-Crosbie, MD, PhD, Massachusetts General Hospital, Boston, Massachusetts, USA, provided background for a discussion of the link between cancer therapy and cardiotoxicity. There are currently > 14 million cancer survivors in the United States, with the National Institutes of Health estimating that 2 million of these are at risk of cardiac events from cancer treatment received. The prevalence of treatment-induced cardiac events is increasing due to a variety of reasons. First, the improved survival of cancer patients is expected to continue to increase the prevalence of cancer survivors, up to 19 million over the next 10 years. Second, cancer survivors tend to be older and carry additional risk factors that compound the risk for cardiac events. Finally, many cancer treatments are becoming more aggressive, involving consecutive courses of multiple drugs, many of which have some defined cardiotoxicity.
In a study of causes of death in breast cancer survivors involving > 63 000 women aged > 65 years, a 9-year median follow-up revealed cardiovascular (CV) disease, not breast cancer, to be the leading cause of mortality (16%) [Patnaik JL et al. Breast Cancer Res. 2011]. Aging was associated with an increasing proportion of CV death within each stage of cancer. Dr Scherrer-Crosbie also addressed the consequences of physicians’ decisions to withhold treatment based on cardiotoxicity side effects. She showed data demonstrating that interruption of trastuzumab for > 1 cycle, due to cardiotoxicity concerns, was associated with a 16% increased chance of breast cancer recurrence [Wu JC et al. AHA. 2013].
In regard to vasculopathy, Dr Scherrer-Crosbie outlined the cancer treatment–related risk factors: anterior or left chest irradiation, high cumulative dose (> 30 Gy), younger age of treatment (< 50 years), high-dose radiation fractions (> 2 Gy/d), presence and extent of tumor in or next to heart, lack of shielding, concomitant chemotherapy (particularly anthracyclines), atherosclerotic risk factors such as diabetes, smoking, obesity, hypertension, and hypercholesterolemia, as well as preexisting CV disease [Lancellotti P et al. Eur Heart J Cardiovasc Imaging. 2013]. In a study of Hodgkin lymphoma survivors, 415 patients with a median age of 25 years at diagnosis were followed up for a median of 11.2 years. Of these survivors, 10.4% developed coronary artery disease (CAD) (diagnosed at a median of 9 years after diagnosis), and 7.4% developed carotid stenosis (diagnosed at a median of 17 years after diagnosis) [Hull P. JAMA. 2003].
In summarizing, Dr Scherrer-Crosbie distinguished between the etiologies of vasculopathy (and valvulopathy) and heart failure/left ventricular (LV) dysfunction, saying that the former is mainly caused by radiotherapy and usually occurs after a long delay. In the case of heart failure/LV dysfunction, anthracyclines increase the risk of cardiac heart failure by 2% to 5%, with the addition of trastuzumab further increasing the risk [Swain S. Cancer. 2003]. Dr Scherrer-Crosbie urged increased collaboration between cardiologists and oncologists on decisions regarding whether to stop cancer treatment due to cardiotoxicity concerns.
Recommendations to Monitor Cardiotoxicity After Chemotherapy
Juan Carlos Plana, MD, Baylor College of Medicine, Houston, Texas, USA, presented the expert consensus statement from the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) on multimodality imaging evaluation of adult patients after chemotherapy [Plana JC et al. J Am Soc Echocardiogr. 2014].
Defining cancer therapeutics–related cardiac dysfunction (CTRCD) as a decrease in left ventricular ejection fraction (LVEF) to a value > 10% below the normal reference value (ejection fraction, 53%), Dr Plana said that CTRCD can also be categorized as symptomatic or asymptomatic, as well reversible or irreversible. There are 2 types of CTRCD. Type I CTRCD is characterized by cellular death, biopsy changes, and permanent damage and is related to cumulative dose. The chemotherapy most associated with type I CTRCD is anthracyclines (eg, doxorubicin). Type II CTRCD is characterized by cellular dysfunction, lack of biopsy changes, not being related to cumulative dose, and reversibility. Type II CTCRD is associated with therapy from monoclonal antibodies such as trastuzumab, pertuzumab (both target epidermal growth factor receptor 2), and bevacizumab (targets vascular endothelial growth factor receptor) but also can result from use of tyrosine kinase inhibitors such as lapatinib, sorafenib, and sunitinib, as well as the proteasome inhibitor bortezomib.
The consensus statement calls for evaluation of LVEF by 3D (preferred) or 2D echocardiography (use of contrast should be considered) and measurement of global longitudinal strain (GLS) prior to trastuzumab for treatment of breast cancer. If LVEF is < 53%, GLS is below the lower limit of normal (LLN), and troponins are elevated, a cardiology consult is recommended. If LVEF is > 53%, GLS is above the LLN, and troponins are normal, repeat the study every 3 months during therapy. With use of this guideline in a small study of LV function in breast cancer patients, LVEF measured at the completion of anthracyclines was not significantly predictive of later cardiotoxicity (P = .075), while peak GLS was predictive of cardiotoxicity (P = .0003) [Cheng S et al. J Am Soc Echocardiogr. 2013].
Recommendations to Monitor Cardiotoxicity After Radiation Therapy
Raluca Dulgheru, MD, University of Liège, Liège, Belgium, presented the expert consensus from the EACVI and the ASE on multimodality imaging evaluation of adult patients after radiotherapy [Lancellotti P et al. Eur Heart J Cardiovasc Imaging. 2013]. As with chemotherapy, there are a large number of cancer survivors who have undergone radiotherapy. Cardiotoxicity from radiotherapy often presents relatively late, perhaps 5 to 20 years after exposure. In childhood cancer survivor studies, incidence of cardiac mortality was seen to continue to increase even 30 years after radiotherapy [Anderson JL et al. JACC. 2007]. The number of patients at risk of developing radiation-induced heart disease (RIHD) is likely to increase, as currently 40% of radiation-treated cancer survivors are ≥ 10 years past their therapy.
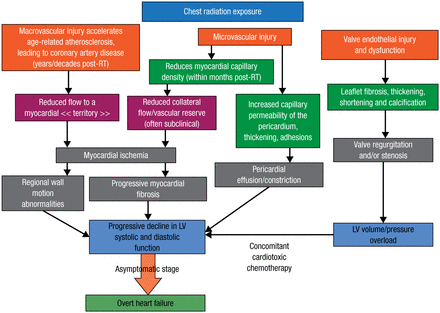
Prof Dulgheru mentioned that several observational studies have revealed the adverse impacts of RIHD. Clinicians should know the ways in which RIHD manifests and that high-risk patients, particularly those who experienced anterior/left chest irradiation, are more likely to benefit from screening. He detailed the mechanisms of RIHD and its consequences following chest radiation exposure in terms of macrovascular and microvascular injury, as well as valve endothelial damage and dysfunction (Figure 1) [Lancellotti P et al. Eur Heart J Cardiovasc Imaging. 2013].
Mechanisms of Radiation-Induced Heart Disease Following Chest Radiation
LV, left ventricular; RT, radiotherapy.
Adapted from Lancellotti P et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2013;14:721-740. By permission of Oxford University Press, on behalf of the European Society of Cardiology and the Authors.
Prof Dulgheru recommended that cardiologists conduct a yearly targeted clinical history and physical examination of patients late after radiotherapy. Key things to know are what type of cancer was involved, how long ago the radiation was, and the patient’s age at the time of therapy. Additionally, questions to answer may include the following: Did the patient have heart disease before radiation? What atherosclerotic risk factors were present at the time of radiation? What cardiac risk factors are currently present, and are they being managed correctly? Prof Dulgheru encouraged clinicians to obtain a full report for the patient from the radiotherapy department to find out if the heart was exposed, the dose per day, and the cumulative dose.
Magnetic Resonance Imaging and CT: How and When to Use Them
In the final seminar on cardiac oncology, Thor Edvardsen, MD, PhD, Oslo University Hospital, Oslo, Norway, delivered a talk on best practices in the use of magnetic resonance imaging and computed tomography (CT) in evaluating cardiotoxicity resulting from cancer therapy. Prof Edvardsen said that cardiac magnetic resonance imaging (CMR) is the reference standard for determining LV and right ventricular volumes and function, as well as myocardial viability. In comparison, CMR enabled reductions in sample size ranging from 71% to 93% relative to echocardiography in detecting clinically significant (90% power and alpha error of 0.05) changes in end-diastolic and end-systolic volumes, ejection fraction, and LV mass [Grothues F et al. Am J Cardiol. 2002]. While CMR does not involve radiation exposure, the technique is time-consuming, expensive, and of somewhat limited availability. Nevertheless, CMR also detects pericarditis and myocardial scar, can be used when echocardiographic quality is suboptimal, and, because of the low sample volume requirements, is ideal for follow-up studies.
Regarding CT, Prof Edvardsen first addressed the visualization of calcification in CAD, acknowledging that there is an uncertain role for CT in imaging coronary arteries before treatment. If treatment-related cardiac symptoms occur, CT may be used to diagnosis atherosclerosis and/or significant stenosis of the coronary arteries. While CT involves radiation exposure, the amount can be limited; the technique is quick; and it is widely available. Similar to CMR, CT can be used to visualize the pericardium and a pericardial effusion, and it is effective in imaging the aorta. Echoing a theme throughout the session, Prof Edvardsen said that cooperation between cardiologists and oncologists is essential to identify and appropriately manage cardiotoxicity arising from cancer treatment and that key clinical decisions should be made only after careful consideration of the available options. Prof Edvardsen encouraged his colleagues to follow these best practice recommendations on a regular basis to engrain the approach.
In conclusion, Prof Edvardsen said that, despite the topic of his talk being CMR and CT, echocardiography is still the first choice for cardiac imaging in the setting of cancer treatment. CMR should also be considered when appropriate, as it is the reference standard for many of the key measurements used in evaluation of possible cardiotoxicity. As a final recommendation, he said that the same techniques should be performed during both baseline assessment and follow-up studies.
- © 2014 SAGE Publications