Summary
The treatment of non-small cell lung cancer (NSCLC) presents several challenges. Practice guidelines are based on limited evidence, and are often unclear and subject to multiple interpretations. Determining when mediastinal lymph nodes should be sampled, and how to best treat stage III disease, presents a particular challenge. Better treatment outcomes are needed.
- Oncology Guidelines
- Pulmonary Guidelines
- Cancer
- Respiratory Cancers
- Oncology Guidelines
- Featured Meeting - Specialty page
- Pulmonary Guidelines
- Pulmonary & Critical Care
- Cancer
- Respiratory Cancers
The treatment of non-small cell lung cancer (NSCLC) presents several challenges. Practice guidelines are based on limited evidence, and are often unclear and subject to multiple interpretations. Determining when mediastinal lymph nodes should be sampled, and how to best treat stage III disease, presents a particular challenge. Better treatment outcomes are needed.
MEDIASTINAL SAMPLING
Anil Vachani, MD, Abramson Cancer Center, Philadelphia, Pennsylvania, USA indicated that positron emission tomography (PET) is the preferred imaging method for mediastinal staging since it provides more accuracy than computed tomography (CT). However, a biopsy is still needed to confirm PET findings. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive technique for obtaining tissue for staging, with results equivalent to those for surgical staging. The 3rd edition of the American College of Chest Physicians Evidence-Based Clinical Practice Guidelines on Lung Cancer (LCIII guidelines) indicate that surgical biopsy should be done if the findings of a needle biopsy are negative [Silvestri GA et al. Chest 2013].
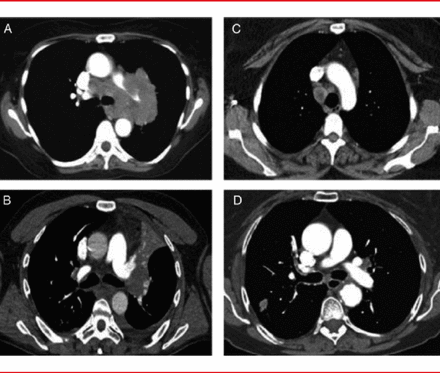
Due to variation in the patterns of lymph node involvement, it is difficult to know when mediastinal sampling is necessary. There are four main patterns of mediastinal lymph node involvement (Figure 1):
-
▪ Massive mediastinal infiltration
-
▪ Discrete node involvement
-
▪ Central tumor with enlarged N1 lymph nodes
-
▪ Peripheral tumor with no mediastinal or hilar node enlargement
The risk of node involvement is high in the first two patterns, so the LCIII guidelines recommend that the nodes be sampled using the easiest method available (Table 1). Dr. Vachani added that the EBUS-TBNA technique allows for the assessment of contralateral hilar disease.
The last two patterns are often classified as a “normal mediastinum” and present greater challenges in staging. For a central lung cancer with enlarged N1 lymph nodes, the risk of mediastinal node involvement is estimated to be 20% to 25%. Dr. Vachani noted that even if PET findings are negative, that risk is high enough to warrant sampling. However, PET is still useful, as it provides a “roadmap” for sampling.
Four Main Patterns of Node Involvement
(A) massive mediastinal Infiltration, (B) discrete node involvement, (C) central tumor with enlarged N1 lymph nodes, and (D) peripheral tumor with no mediastinal or hilar node enlargement.
Reproduced from Silvestri GA et al. Methods for Staging Non-small Cell Lung Cancer. Chest 2013;145(5)Suppl. With permission from the American College of Chest Physicians.
For a peripheral tumor with radiographic evidence of mediastinal or hilar node enlargement, the percentage of N2 or N3 disease ranges from 2% to 9%. When the findings of both CT and PET are negative, the prevalence of N2 or N3 disease decreases to ∼4%. The prevalence of node involvement increases with the size of the tumor; the prevalence of N2 or N3 disease increases to 13% for T2 lesions.
Dr. Vachani also noted that the LCIII recommendation addresses lesions that are either T1a or T1b, and does not provide specific guidance for larger primary lesions with a normal mediastinum. He added that mediastinal sampling should be considered when the prevalence of mediastinal disease is >10%.
OPTIMAL TREATMENT OF STAGE III DISEASE
One of the greatest challenges in determining the best treatment for stage III NSCLC is that the population of patients is highly heterogeneous, said Douglas Arenberg, MD, University of Michigan, Ann Arbor, Michigan, USA. Stage III NSCLC represents the largest proportion of available stages, with 25 TNM combinations being classified as stage III in the 7th edition of the American Joint Committee on Cancer Staging Manual.
Focusing on identifiable stage III NSCLC at the time of diagnosis (excluding clinically occult N2 disease), Dr. Arenberg noted that the goal of treatment is to eradicate gross chest disease and prevent distant metastasis, which is a common cause of death. Due to poor survival rates, radiation therapy or surgery alone are no longer considered first-line treatment options. Poor surgical outcomes are primarily related to a high proportion of incomplete resections, even among patients treated by experienced teams. However, pooled data have shown that (for patients with unexpected stage III disease discovered at the time of surgery, after thorough preoperative staging) cisplatin-based adjuvant chemotherapy is associated with a benefit in overall survival (compared with surgery alone) for stage I-III NSCLC, and the benefit is greatest for people with stage III disease [Pignon JP et al. J Clin Oncol 2008].
Chemoradiation therapy as definitive treatment or as induction treatment (followed by planned surgery) has also led to slightly higher 5-year survival rates, especially when chemotherapy and radiation therapy are given concurrently. Studies have shown that both preoperative chemoradiation and definitive chemoradiation therapy lead to similar outcomes, with average 5-year survival rates of 19% and 22%. These findings led to the LCIII recommendation of either definitive chemoradiation therapy or induction therapy followed by surgery for patients with stage III NSCLC (Table 1) [Ramnath N et al. Chest 2013]. Patients in whom complete resection is not feasible should be treated with chemoradiation therapy.
LCIII Recommendations for Mediastinal Sampling of Early-Stage NSCLC and Treatment of Stage III NSCLCa
Dr. Arenberg also emphasized that it is not possible to identify patients who are more likely to benefit from surgical resection after induction therapy based on pretreatment characteristics. He emphasized several points:
-
▪ All treatment recommendations are based on the assumption that staging has been thoroughly carried out and is unequivocal.
-
▪ It is essential to plan all treatments upfront rather than to “see what happens with chemotherapy and radiation therapy first.”
-
▪ Treatment must be planned by a multidisciplinary team; this team should also track relevant clinical outcomes.
-
▪ Patient preferences are paramount in decision-making.
-
▪ Toxicity and complications should be minimized and well-managed.
ROLE OF TARGETED THERAPY
With metastasis as the most common cause of systemic failure, is there a role for targeted therapy in adjuvant treatment? Gregory J. Riely, MD, Memorial Sloan-Kettering Cancer Center, New York, New York, USA, discussed the ongoing trials that are addressing this question. The tyrosine kinase inhibitors (TKI) erlotinib and gefitinib have been evaluated as targeted therapy for NSCLC that tests positively for the EGFR mutation (EGFR+).
Studies have shown that these targeted agents extend progression-free survival (compared with chemotherapy alone) for patients with metastatic EGFR+ NSCLC [Maemondo M et al. N Engl J Med 2010; Rosell R et al. Lancet Oncol 2012]. Erlotinib and gefitinib are now being evaluated in the adjuvant setting. One Phase 2 trial indicated that adjuvant erlotinib for early-stage EGFR+ NSCLC is feasible and is associated with disease-free survival at 2 years (94%) [Neal JW et al. J Clin Oncol 2012]. In another retrospective study, D'Angelo and colleagues show that the two targeted therapy agents led to longer disease-free and overall survival among patients with early-stage, resected EGFR+ NSCLC [J Thorac Oncol 2013]. Further studies are needed to determine whether an EGFR TKI has a role as part of adjuvant treatment of early-stage EGFR+ NSCLC.
- © 2013 MD Conference Express®