Summary
Prior to the 20th century, fungal diseases were rare in humans; however, between 1899 and 1999 fungal diseases such as Candida albicans, Coccidiodes immitis, and Aspergillus spp. became distressingly common. The introduction of penicillin in the 1940s changed human flora, and a few years later plastic catheters, which provide microbial access ports, were introduced. Then followed the introduction of intensive care units, organ transplants, and in the late 1980s, the HIV pandemic. All of these together worked to transform “nonpathogens” into “pathogens.”
- Featured Meeting - Specialty page
- Fungal Infections
Prior to the 20th century, fungal diseases were rare in humans; however, between 1899 and 1999 fungal diseases such as Candida albicans, Coccidiodes immitis, and Aspergillus spp. became distressingly common. “What happened?” asked Arturo Casadevall, MD, PhD, Albert Einstein College of Medicine, Bronx, New York, USA. His answer was, “We changed the host.” The introduction of penicillin in the 1940s changed human flora, and a few years later plastic catheters, which provide microbial access ports, were introduced. Then followed the introduction of intensive care units, organ transplants, and in the late 1980s, the HIV pandemic. All of these together worked to transform “nonpathogens” into “pathogens.”
Pathogenic microbes can be divided into 2 categories, depending on the source of infection: host-acquired (disease results from a disruption of host-microbe relationship) and environment-acquired (disease results from a host with impaired immunity or large inoculum). With the exception of Candida and the dermatophytes, most fungal pathogens are environmentally acquired and are the only known group of pathogens that can drive a species to extinction.
Cryptococcus neoformans (Cn), a soil fungus that causes life-threatening meningitis in immunocompromised patients and is a facultative intracellular pathogen capable of replication inside macrophages, is of particular research interest to Dr. Casadevall. A unique aspect of Cn's interaction with macrophages is the phenomenon of nonlytic exocytosis, which involves the escape of fungal cells from the phagocyte with the survival of both cell types [Nicola AM et al. MBio 2011]. Cn is also particularly interesting in that it can also cause disease in plants, insects, and protozoa. Melanin exists on the outside wall of cells to protect against damage from insults such as UV light and heat. Melanized fungal cells have also been shown to demonstrate increased growth relative to nonmelanized cells after exposure to ionizing radiation [Dadachova E et al. PLoS One 2007], raising questions about a potential role for melanin in energy capture and utilization [Zhdanova NN et al. Mycol Res 2000]. Research suggests that the virulence of Cn for mammalian cells is a consequence of adaptations that have evolved for protection against environmental predators such as amoebae and provides an explanation for the broad host range of this pathogenic fungus [Steenbergen JN et al. Proc Natl Acad Sci USA 2001].
Shifting to an historical perspective, Dr. Casadevall noted that most of Earth's recent history has been dominated by reptiles. But something shifted 65 million years ago when ∼70% of all species then living on Earth disappeared within a very short period. What followed was a dramatic increase in mammalian megafauna and a decline in reptilian megafauna.
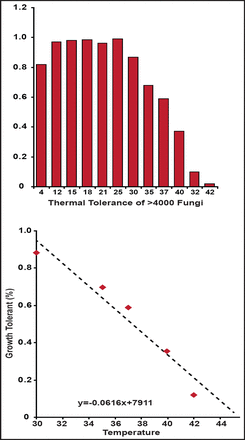
Relative to reptiles, mammals require more energy to survive and produce fewer offspring that require considerable care. If reptiles are so fit, why did they not experience a resurgence [Hulbert AJ, Else PL. Am J Physiol 1981]? Dr. Casadevall suggested that a fungal filter selected for mammals over reptiles led to the age of mammals. This theory is supported by data showing that most fungal strains cannot grow at mammalian temperatures (Figure 1) [Robert VA, Casadevall A. J Infect Dis 2009].
Temperature Tolerances for Fungi.
Robert A & Casadevall A. Vertebrate Endothermy Restricts Most Fungi as Potential Pathogens. J Infect Dis. (2009) 200 (10): 1623–1626 with permission from Oxford University Press.
Mammalian endothermy enhances fitness by creating exclusionary thermal zones that protect against fungal diseases. When the tradeoff involved between the cost of the excess metabolic rates required to maintain body temperature and the benefit gained by creating a thermal exclusion zone that protects against environmental microbes such as fungi is analyzed, the resulting temperature is 36.7°C. A temperature that, in Dr. Casadevall's opinion, is so close to the mammalian temperatures that it is unlikely to be arrived at by coincidence or accident [Bergman A, Casadevall A. MBio 2010].
Bats provide an example of the relationship between temperature and fungal infection. They have a normal body temperature of 37°C, but when they hibernate, their temperature drops to 10°C to 12°C. It is during this period that they become susceptible to white-nose syndrome (caused by Geomyces spp.), which wakes them in the winter and causes them to die. The issue can be resolved in the laboratory by awakening the hibernating bats and feeding them, thereby increasing their body temperature.
Additional support for the temperature theory comes from fossil evidence dating after the meteor impact in the Yucatan Peninsula. This evidence indicates that the resulting fires led to large amounts of smoke and dust, which obscured the Sun and resulted in a shutdown of photosynthesis for ∼6 months. Global temperatures dropped, vegetation died off, and there was massive proliferation of fungi. One hypothesis is that it was the increase in fungal disease to which reptiles are much more susceptible than mammals that caused so many of them to die off [Casadevall A. PLoS Pathog 2012].
Most fungal diseases are from the area around the equator and areas around the planet that are warmer that have allowed the fungi to adapt themselves to warmer temperatures. What has protected humans from fungal diseases has been the distance between the human temperature and the ambient temperature. Dr. Casadevall asked if this means that “as the ambient temperature rises we will see more fungal diseases”? Nonthermal tolerant fungi with pathogenic potential can adapt to higher temperatures. In 1 study that used a newly developed automated continuous culture that takes advantage of a natural selection-adaptation strategy, 2 thermotolerant variants of Metarhizium anisopliae displayed robust growth at 36.5°C. Thermotolerant variants were entomopathogenic, albeit with complex alterations in virulence parameters such as lethal dose responses and median survival times. Thus raising the possibility that fungus will adapt to survive at higher temperatures as the world becomes warmer [de Crecy E et al. BMC Biotechnol 2009].
Dr. Casadevall predicted that as the 21st century progresses, fungal disease will increase, new fungal pathogens will emerge, and new pathogenic genera will emerge—some of which will be resistant to current drugs. As a result, some mammals may be in trouble.
- © 2012 MD Conference Express®