Summary
This article discusses the rationale for using methyl aminolevulinate cream (MAL) and exposure to sunlight (daylight photodynamic therapy) as an alternative for MAL and conventional photodynamic therapy to treat actinic keratoses.
- Dermatology Clinical Trials
- Skin Cancer
- Soft Tissue Cancers
- Dermatology
- Dermatology Clinical Trials
- Skin Cancer
- Soft Tissue Cancers
Rolf-Markus Szeimies, MD, PhD, Klinikum Vest Academic Teaching Hospital, Recklinghausen, Germany, discussed the rationale for using methyl aminolevulinate cream (MAL) and exposure to sunlight (daylight photodynamic therapy [DL-PDT]) as an alternative for MAL and conventional photodynamic therapy (c-PDT) to treat actinic keratoses (AKs). Although AKs of the scalp and face respond very well to c-PDT, the procedure is associated with pain and inconvenience [Rubel DM et al. Br J Dermatol. 2014; Kennedy JC, Pottier RH. J Photochem Photobiol B. 1992]. Previously published data from a series of studies [Wiegell SR et al. Br J Dermatol. 2012; Wiegell SR et al. Br J Dermatol. 2011; Wiegell SR et al. Br J Dermatol. 2009; Weigell SR et al. Br J Dermatol. 2008] and an international consensus paper [Wiegell SR et al. J Eur Acad Dermatol Venereol. 2012] all suggest that even in Scandinavian countries, DL-PDT is an effective, safe, and more convenient alternative to MAL-PDT as a treatment for many people with AKs.
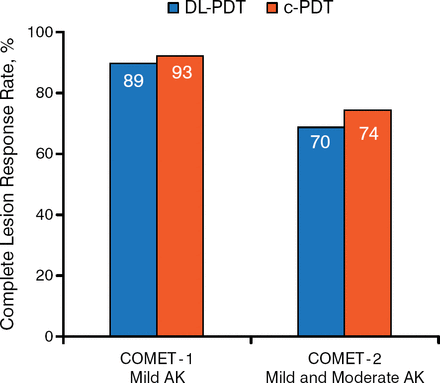
Prof Szeimies reviewed data from the Intra-individual Comparison of Efficacy and Safety of Metvix Natural Daylight Photodynamic Therapy Versus Conventional Metvix Photodynamic Therapy in Subjects With Mild Actinic Keratoses trial [COMET-1; Rubel DM et al. Br J Dermatol. 2014] and the Phase 3b Study of Metvix NDL-PDT Versus Metvix c-PDT in Subjects With Actinic Keratoses [COMET-2; NCT01821391]. Both were randomized, phase 3, noninferiority studies of patients with mild (COMET-1) or mild-to-moderate (COMET-2) AKs.
COMET-1 included 100 Australian adults, and COMET-2 included 108 European patients. In both trials, patients were randomized to a single treatment of DL-PDT to either side of the face and c-PDT plus sunscreen to the other side. They were all followed for an initial 12 weeks; lesions that responded completely after the first 12 weeks were then followed for an additional 12 weeks. The primary efficacy outcome was complete AK lesion response rate per side at week 12. The primary safety end point was the self-reported pain assessment using the Visual Analog Scale (VAS) just after the treatment session at the baseline visit.
In both trials, the majority of patients were men. All were white with a mean age ranging from 67 to 73 years. The mean number of lesions on both sides at baseline in COMET-1 was 14, and it was 9 in COMET-2. At week 12, DL-PDT was noninferior to c-PDT in either trial (Figure 1). In COMET-1, 97% of the lesions that had completely responded at week 12 remained clear after 24 weeks; data were not presented for COMET-2. Prof Szeimies noted that DL-PDT was effective in either sunny or cloudy weather, with a similar rate of complete lesion response at 3 months after 1 session, but emphasized that the procedure will not be successful in rainy weather.
Primary Efficacy End Point at Week 12
AK, actinic keratosis; c-PDT, conventional photodynamic therapy; DL-PDT, daylight photodynamic therapy.
Reproduced with permission from RM Szeimies, MD, PhD.
Prof Szeimies went on to discuss the primary safety end point. The VAS scores were 0.8 and 7.7 (P < .001) with DL-PDT and c-PDT, respectively, in COMET-1 and were 0.7 and 4.4 (P < .001), respectively, in COMET-2. In COMET-1 and COMET-2, 82% and 91% of patients respectively treated with DL-PDT did not report any pain. In COMET-1, patients were more highly motivated to consider retreatment with DL-PDT than the conventional treatment (93% vs 63%); data were not available for COMET-2.
In summary, data from Australia and Europe suggest that DL-PDT is noninferior to c-PDT in treating mild and moderate AK and that the efficacy is sustained throughout at least 24 weeks for either procedure. Patients reported less pain when they underwent DL-PDT, expressed satisfaction with the procedure, and were motivated to seek out retreatment if necessary.
- © 2014 MD Conference Express®