Summary
This article presents the results of a multicenter study of downstaging of hepatocellular carcinoma to within the Milan staging criteria before liver transplantation, demonstrating excellent posttransplantation outcomes.
- Gastrointestinal Cancers
- Transplantation Hepatology Clinical Trials
- Cancers of the Accessory Digestive Organs
- Gastrointestinal Cancers
- Transplantation
- Hepatology
- Hepatology Clinical Trials
- Cancers of the Accessory Digestive Organs
Neil Mehta, MD, University of California San Francisco, San Francisco, California, USA, presented the results of a multicenter study of downstaging of hepatocellular carcinoma (HCC) to within the Milan staging criteria before liver transplantation (LT), demonstrating excellent posttransplantation outcomes.
According to Dr Mehta, the Milan criteria represent the gold standard to select candidates for LT [Mazzaferro V et al. N Engl J Med. 1996]. He explained that downstaging of HCC represents a selection strategy involving expanded transplant criteria based on the control of tumor growth by locoregional therapy (LRT).
Currently in the USA, patients with HCC are only eligible for priority listing for LT with model for end-stage liver disease (MELD) exception if they meet stage T2 criteria.
Although downstaging to within Milan criteria has been shown to produce favorable post-LT outcomes in single-center studies, no multicenter studies have previously been reported. Consequently, this multicenter study aimed to evaluate post-LT and intention-to-treat outcomes under a uniform Region 5 downstaging protocol [Yao FY et al. Hepatology. 2008]. Successful downstaging was defined by having residual tumor within the Milan criteria.
The study enrolled 187 consecutive adult patients with HCC from 3 centers who were treated under the Region 5 downstaging protocol. Patients were included if they had 1 lesion > 5 cm and ≤ 8 cm; 2 or 3 lesions, each ≤ 5 cm, with the total diameter of all ≤ 8 cm; 4 or 5 lesions, each ≤ 3 cm, with the total diameter of all ≤ 8 cm; and no vascular invasion evident on imaging.
Of those who initially enrolled, 36.4% (n = 68) experienced dropout due to tumor progression or death at a median of 8 months from the first downstaging procedure. The probability of dropout from first downstaging was 26% at 1 year and 41% at 2 years, and the only significant predictors of dropout were Child-Pugh class B (P = .02) and C (P = .005) disease.
Successful downstaging was experienced by 63.6% (n = 119) of patients. Of these, 106 underwent deceased donor LT (DDLT), 3 underwent living donor LT, and 10 were awaiting DDLT at the end of study follow-up. The median time from first downstaging to LT was 12.6 months, with a median post-LT follow-up of 4.3 years.
Tumor characteristics were favorable at explant: 81% of patients overall were within Milan criteria, and 35% had complete tumor necrosis; of 71 patients with residual viable tumor, only 1 had a poorly differentiated tumor; and overall, only 6% of patients had microvascular invasion.
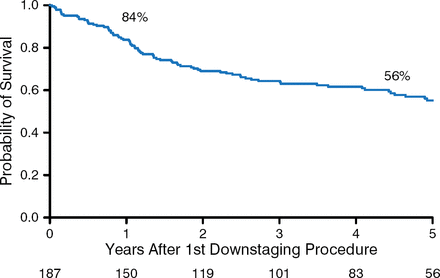
For the entire cohort, intention-to-treat survival was 84% at 1 year and 56% at 5 years (Figure 1).
Intention-to-Treat Survival Following Liver Transplantation
Reproduced with permission from N Mehta, MD.
Post-LT survival at was 95% at 1 year and 80% at 5 years. Of the 109 patients who underwent LT, 11% experienced HCC recurrence at a median of 19.1 months from transplantation.
The overall recurrence-free probability was 95% at 1 year, and 87% at 5 years from transplantation. The only significant predictors of HCC recurrence were alpha-fetoprotein > 500 ng/mL (P = .003) and microvascular invasion (P = .002). No center-specific differences seen in intention-to-treat survival, post-LT survival, and overall recurrence-free probability of the cohort.
Data from this largest study to date, and the first multicenter study, demonstrated successful downstaging of HCC to within the Milan criteria in almost two-thirds of patients. These results support broader application of this uniform downstaging protocol, concluded Dr Mehta.
- © 2014 MD Conference Express®