Summary
Rectal cancer is treated with surgery, radiation therapy (RT), and neoadjuvant and adjuvant chemotherapy (CT); yet, the best way to sequence and combine these modalities is not yet known. This article discusses organ-sparing surgery in rectal cancer, the feasibility of intensifying preoperative RT, as well as preoperative therapy.
- Adjuvant/Neoadjuvant Therapy
- Gastrointestinal Cancers
- Reproductive Cancers
- Oncology
Rectal cancer is treated with surgery, radiation therapy (RT), and neoadjuvant and adjuvant chemotherapy (CT); yet, the best way to sequence and combine these modalities is not yet known.
Andre D'Hoore, MD, PhD, Catholic University of Leuven, Leuven, Belgium, discussed organ-sparing surgery in rectal cancer. Screening programs are detecting more early rectal cancers, and practitioners are increasingly aware that radical surgery, such as total mesorectal excision (TME), causes short- and long-term functional morbidity. Neoadjuvant chemoradiation therapy (CRT) results in significant tumor downstaging, and new surgical techniques to perform local excision (LE) are available to remove smaller tumors, including those found during transanal endoscopic microsurgery.
The advantages of LE in early rectal cancer include minimal perioperative mortality and morbidity, rapid recovery, sphincter preservation and avoidance of a permanent colostomy, preservation of bowel and urogenital function, improved quality of life, and reduction in healthcare costs. However, one disadvantage of LE is that the lymph nodes (LN) are not sampled, unlike during radical resections, so the recurrence rate after LE is higher. If the tumor histology is unfavorable after LE, a completion radical resection can be performed. LE can be curative in early rectal cancers that are minimally invasive into the submucosa and are well to moderately differentiated without lymphovascular invasion. Patients who undergo LE should be followed up using endoscopy and imaging with magnetic resonance (MRI) and positron emission tomography (PET), although the optimal examination interval and follow-up duration is not clear.
Karin Haustermans, MD, PhD, University Hospitals Leuven, Leuven, Belgium, discussed the feasibility of intensifying preoperative RT in patients with rectal cancer and addressed methods to intensify preoperative RT. Whereas preoperative RT followed by TME is the standard treatment for locally advanced stages of rectal cancer, the response to preoperative RT is not always the same. Indeed, up to 27% of patients will experience pathological complete remission and these patients could be considered for organ preservation rather than TME. Organ preservation is appealing because it avoids long-term genitourinary and fecal complications and postoperative mortality and morbidity, and provides a better increasing quality of life. Evidence suggests that the oncologic outcomes of this approach are satisfactory as well [Maas M et al. J Clin Oncol. 2011; Habr-Gama et al. Ann Surg. 2004].
The remaining patients will likely undergo treatment intensification. Prof. Haustermans emphasized that intensifying RT is a local therapy, and that the target of the radiation should fall outside of the surgical margins. She then went on to discuss 2 different methods of dose escalation.
The Lyon R96–02 study [Gerard J-P et al. J Clin Oncol. 2004], a Phase 3 dose-escalation randomized trial compared external beam RT (EBRT; n = 43) with EBRT plus contact RT boost (n = 45) in patients with T2 or T3, Nx, or M0 stage of rectal cancer. EBRT plus boost significantly reduced tumor diameter (p = .03) and significantly increased the use of sphincter-saving surgery (p = .004). Surgical complications and acute toxicity were comparable for both groups.
Another study examined the optimal time after neoadjuvant CRT to assess tumor response in 91 patients with cT2–4N0–2M0 distal rectal adenocarcinomas [Perez RO et al. Int J Rad Oncol Biol Phys. 2012]. Tumors with an increase in standardized uptake value (SUVmax), as determined by PET/computed tomography (CT) between 6 and 12 weeks, were less likely to develop significant downstaging after CRT. Decreased SUVmax at the 6-week PET/CT examination predicted good response. SUVmax variations could be used to determine CRT—surgery intervals for patients.
David Cunningham, MD, Royal Marsden Hospital, London, United Kingdom, discussed intensification of preoperative therapy. Prof. Cunningham believes that a plateau has been reached in survival of patients with locally advanced T3 or T4, node-positive rectal cancer after preoperative CRT, surgery, and postoperative CT, and that distal recurrence is the primary cause of treatment failure and death. Therefore, new treatment strategies are required, and intensifying CT may be required for some patients. This necessitates accurate preoperative evaluations and staging to determine which patients may benefit from this approach.
Preoperative systemic CT is better tolerated than adjuvant CT, and it permits the evaluation of tumor sensitivity to CT within the patient; treats micrometastases early, so it potentially improves survival; and may limit the need for RT and spare the patient the related toxicity [Fernandez-Martos C et al. J Clin Oncol. 2010]. Neoadjuvant CT followed by CRT is feasible and effective [Chua YJ et al. Lancet Oncol. 2010; Dewdney A et al. J Clin Oncol. 2013]. The results of these Phase 2 trials warrant further study.
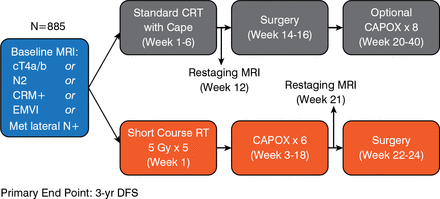
The Rectal Cancer and Preoperative Induction Therapy Followed by Dedicated Operation trial [RAPIDO; Nilsson PJ et al. BMC Cancer. 2013] will determine if neoadjuvant CT after short-course RT may be an alternative option. The primary end point is 3-year disease-free survival. The schema is shown in Figure 1.
RAPIDO Trial Schema
cT=clinical tumor; Cape=capecitabine; CAPOX=capecitabine plus oxaliplatin; CRM=circumferential resection margin; CRT=chemoradiotherapy; EMVI=extramural vascular invasion; Gy=Gray; Met=metastatic; MRI=magnetic resonance imaging; N=node; RT=radiotherapy.
Adapted from Nilsson PJ et al. BMC Cancer. 2013.
There are many open questions concerning intensification of preoperative treatment with systemic therapy, including the role of targeted therapies, whose role in rectal cancer is still controversial, in part because predictive biomarkers for rectal cancer are lacking. Currently, patients with very advanced, high-risk rectal cancer are excluded or under-represented in clinical trials and need better therapy.
The results of ongoing trials to optimize radiation doses and fractionation, to determine the time interval from neoadjuvant CRT to surgery, and to identify intensified RT and CT regimens will help personalize rectal cancer treatment and reduce the risk of recurrence. Research to identify predictive biomarkers for conventional, targeted, and intensified therapies for patient selection is crucial.
- © 2014 MD Conference Express®