Summary
The pandemic of metabolic disease caused by the increase in obesity is the greatest current threat to human health. At this time, 1.2 billion people worldwide are overweight or obese, and 400 million have been diagnosed with diabetes. This article discusses the integration of nutrients, immune response, and metabolism in health and disease.
- obesity
- inflammatory disease
Gökhan S. Hotamisligil, MD, PhD, Harvard School of Public Health, Boston, Massachusetts, USA, presented the Danone International Prize for Nutrition Laureate lecture on the integration of nutrients, immune response, and metabolism in health and disease. According to Dr. Hotamisligil, the pandemic of metabolic disease caused by the increase in obesity is the greatest current threat to human health. At this time, 1.2 billion people worldwide are overweight or obese, and 400 million have been diagnosed with diabetes [International Diabetes Federation. IDF Diabetes Atlas: Third Edition, 2006. http://www.idf.org/atlasmap/atlasmap].
IMMUNE RESPONSE AND METABOLIC DISEASE
In 1943, Greene and Keohen suggested that infection was among the common causes of insulin resistance. Forty years later, Pekala, Cerami, and colleagues demonstrated that adipocytes develop insulin resistance when exposed to supernatants of endotoxin-treated macrophages. In addition, the researchers described the possible mechanism that involved defects in postreceptor insulin signaling [Pekala et al. J Exp Med 1983].
In 1993, Dr. Hotamisligil and colleagues found that inflammatory cytokines such as tumor necrosis factor alpha (TNF-a) are expressed in the white adipose tissue of obese mice. In 1995, two studies showed the same phenomenon in obese humans but not in lean individuals. Subsequent studies genetically linked TNF-a with insulin resistance and showed that TNF-a inhibition restored insulin function.
Dr. Hotamisligil and others have since shown that >50 cytokines, chemokines, and inflammatory mediators are associated with obesity and linked to insulin resistance and diabetes. Other studies have focused on the identification of key signaling molecules and triggering mechanisms to develop inhibiting therapies. Among these were studies showing that the c-Jun amino-terminal kinases (JNKs) are abnormally elevated and mediate insulin resistance and diabetes in obesity [Bluher M et al. J Clin Endocrinol Met 2009; Boden G et al. Diabetes 2009; Hirosumi J et al. Nature 2002]. Numerous studies have shown that JNK activation in adipose tissue, muscle, macrophages, β cells, and the brain impairs metabolism [Han MS et al. Science 2013; Lanuza-Masdeu J et al. Diabetes 2013; Henstridge DC et al. Diabetologia 2012; Zhang X Diabetes 2011; Belgardt BF et al. Proc Natl Acad Sci USA 2010]. Additionally, pharmacologic JNK inhibition has been shown to promote metabolic homeostasis [Rondinone CM. Expert Opin Ther Targets 2005; Kaneto H et al. Nat Med 2004]. Other studies identified inhibitory kappa kinase, another central inflammatory signaling molecule as a key component of insulin resistance and diabetes [Yuan et al. Science 2001]. Additional studies have shown that immune cells can infiltrate adipose tissue with obesity. Theses studies, as well as others, have shown that obesity and inflammation are linked.
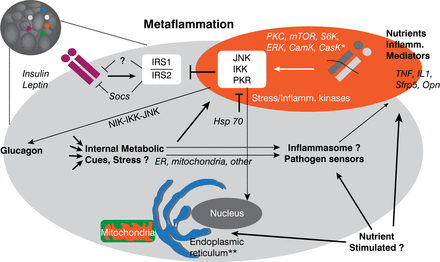
The modified immune response that occurs with metabolic stress is referred to as metabolic inflammation. Also known as metaflammation, this response is characterized by signals and responses from metabolic cells such as adipocytes, interactions between metabolic and immune cells, and alterations in both energy management and insulin action (Figure 1). While inflammation is a critical adaptive response necessary for tissue repair and homeostasis, chronic inflammation is associated with some adverse events.
Cellular Interactions in Metaflammation
Reproduced with permission from GS Hotamisligil, MD, PhD.
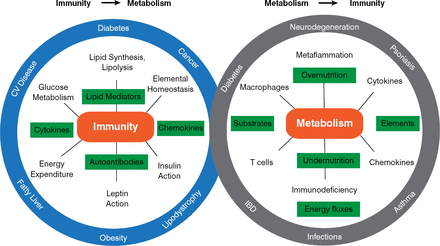
Inflammation in adipose tissue involves macrophages, mast cells, T and B cells, and eosinophils. While adipose tissue is the most studied site of immune response in obesity, other tissues— such as liver, pancreas, and brain—are part of these integrated pathways. Immunometabolism has emerged as new field of study that examines the role of chronic inflammation and stress in metabolic regulation, as well as the influence of metabolism on the immune response. According to Dr. Hotamisligil, immunometabolism has implications for a variety of chronic and metabolic diseases (Figure 2). Dr. Hotamisligil also stressed that the relation between immunity and metabolism is bidirectional. The immune response influences metabolic outcomes, but the metabolic milieu—in immune cells and systemically—also serves as a critical regulator of immune function.
Relationship Between Immunity and Metabolism
Reproduced with permission from GS Hotamisligil, MD, PhD.
ENDOPLASMIC RETICULUM STRESS AND DYSFUNCTION
The endoplasmic reticulum (ER) is an intracellular organelle that is involved in protein synthesis, folding, trafficking, and quality control. The ER responds to changes in protein, lipid, and carbohydrate synthesis in the cell through 3 molecules in its membrane—protein kinase RNA-like ER kinase, inositol-requiring enzyme 1 (IRE-1), and activating transcription factor 6 (ATF-6), which collectively maintain equilibrium in the ER. Obesity has been shown to disrupt this pathway, both in experimental models and in humans; however, weight loss can reverse this ER stress. The discovery that JNK is involved in activation of the IRE-1 pathway showed that JNK is closely linked to ER function, thereby stimulating further studies to explore the role of ER in obesity and diabetes [Urano et al. Science 2002].
Chronic activation of ER due to stress and a defective response to proteins that have not yet been folded into the ER both play an important role in the development of insulin resistance and diabetes in obesity. Fu et al. [PLoS Genetics 2012; Cell Met 2012; Nature 2011] studied the mechanisms leading to chronic ER stress in obesity. The researchers were able to clearly distinguish lean and obese ER proteomics and lipidomics. This study found increased lipogenesis, decreased protein and RNA synthesis, abnormal ER calcium homeostasis, mitochondrial defects, and altered bile acid metabolism associated with obesity. Alterations in autophagy and proteosome activity have also been found in association with obesity [Yang L et al. Cell Metab 2010]. These and other studies demonstrate that chronic excess energy and nutrients lead to abnormal ER morphology, organization, and function. It also has been shown that the ER can serve as a powerful recipient of nutrient and energy signals in the cells and that it is strongly influenced by the nutritional status of the organism.
Chronic inflammatory pathways are associated with the unfolded protein response, which is activated when the ER is stressed by the accumulation of newly synthesized unfolded proteins [Hotamisligil G. Cell 2010]. The inflammatory processes related to the unfolded protein response, molecular mediators, sensing mechanisms, and the related physiologic outcomes are not completely understood. However, under inflammatory conditions, defects in the expression of the unfolded protein response mediators ATF-6 and X-Box binding protein 1 have been identified in liver as well as in pancreatic β cells from type 1 and type 2 diabetes (T1D and T2D) mouse models and humans [Engin F et al. Sci Transl Med 2013; Sci Reports 2013]. In T1D mice, restoration of the unfolded protein response in β cells by administration of a chemical ER stress mitigator at the pre-diabetic stage resulted in a marked decrease in diabetes incidence, along with decreased pancreatic lymphocyte infiltration, improved β-cell survival and morphology, reduced β-cell apoptosis, preserved insulin secretion, and restored unfolded protein response mediator expression. Benefits of such interventions are also observed in T2D in experimental animal models and in humans [Kars et al. Diabetes 2010; Xiao et al. Diabetes 2011].
Dr. Hotamisligil stated that the molecular links between immune response and unfolded protein response are an area of intense study and that much remains to be discovered. The upstream signals that give rise to JNK activation and elF2a phosphorylation in obesity also are unclear. It is known that the elF2a kinase, a double-stranded RNA-dependent protein kinase, is activated in mouse and human obesity and plays a unique role in unfolded protein response and related inflammatory and metabolic events [Nakamura et al. Cell 2010; Carvalho-Filho et al. Endocrinology 2012; Carvalho et al. Obesity 2013]. Double-stranded RNA-dependent protein kinases also coordinate the activity of JNK and other inflammatory kinases to regulate insulin action and metabolism [Nakamura T et al. Cell 2010]. Studies on this unique signaling molecule offer opportunities to understand which dietary and microbial components may directly engage immune pathways and allow mechanisms linking metabolism and immunity at the molecular level.
TARGETING METAFLAMMATION
According to Dr. Hotamisligil, many challenges lie ahead, but progress has been made in targeting metaflammation. TNF-a antagonism has been shown to decrease glucose levels and increase the proportion of high molecular weight adiponectin levels in obese humans with metabolic syndrome [Stanley TL et al. J Clin Endocrinol Metab 2011]. Anti-TNF-a agents have reduced diabetes incidence by 50% in patients with rheumatoid arthritis and psoriasis in multiple studies [Solomon DH et al. JAMA 2011]. An interleukin-1 receptor antagonist also improved glycemia and β-cell function and reduced markers of systemic inflammation in patients with T2D in multiple independent studies [Larsen CM et al. N Engl J Med 2007]. Glycemia also was improved by salsalate, a prodrug of salicylate, in patients with type 2 diabetes [Goldfine AB et al. Ann Intern Med 2010]. While there are also unsuccessful attempts to utilize these agents in humans and it is yet unclear whether these specific drugs will find broad use in human disease, the field of immunometabolism is expanding faster than any other field, and more creative and effective strategies will surely be identified and applied to human disease.
Dr. Hotamisligil concluded that immunometabolic homeostasis is a delicate balance of forces, including host structure, genetic variation, environmental factors, physical activity, nutrient intake, stress, microbiota structure, circadian cycles, epigenetic effects, and the systemic energetics. Outcomes of health or disease depend on the interactions of these factors with the immune system. In immunometabolism, where multiple pathways are involved, it is unlikely that targeting a single aspect of the pathway will result in effective treatments. Rather, utilization of systematic approaches should teach us how best to modulate this response to promote health and prevent disease.
- © 2014 MD Conference Express®