Summary
The results of the CALGB/SWOG Phase 3 trial [NCT00265850; Venook AP et al. J Clin Oncol 2014] reveal similar outcomes with FOLFIRI (folinic acid, 5-fluorouracil, and irinotecan) or FOLFOX (folinic acid, 5-fluorouracil, and oxaliplatin) chemotherapy combined with cetuximab or bevacizumab as a first-line treatment for select patients with KRAS wild-type metastatic colorectal cancer (mCRC). The similarities mean that patients have treatment choices and can now opt to start treatment with the regimen that they most prefer.
- Gastrointestinal Cancers Clinical Trials
- Oncology
- Gastrointestinal Cancers
- Oncology Clinical Trials
The results of the CALGB/SWOG Phase 3 trial reveal similar outcomes with FOLFIRI (folinic acid, 5-fluorouracil, and irinotecan) or FOLFOX (folinic acid, 5-fluorouracil, and oxaliplatin) chemotherapy combined with cetuximab or bevacizumab as a first-line treatment for select patients with KRAS wild-type metastatic colorectal cancer (mCRC). The similarities mean that patients have treatment choices and can now opt to start treatment with the regimen that they most prefer, according to presenter Alan P. Venook, MD, University of California, San Francisco, California, USA, who presented the results of a Phase 3 trial of FOLFIRI or FOLFOX with bevacizumab or cetuximab for patients with KRAS wild-type untreated metastatic adenocarcinoma of the colon or rectum [CALBG/SWOG 80405; NCT00265850; Venook AP et al. J Clin Oncol 2014].
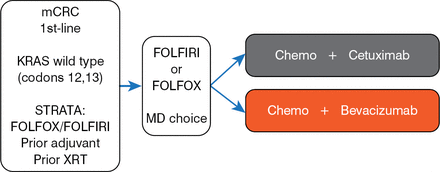
The trial commenced in 2004 and took 10 years to complete. The final design of the trial, in 2008 (Figure 1), reflected an amendment process due to advances in the knowledge of advanced colorectal cancer (CRC).
CALGB/SWOG 80405 Final Design
chemo=chemotherapy; FOLFIRI=folinic acid, 5-fluorouracil, and irinotecan; FOLFOX=folinic acid, 5-fluorouracil, and oxaliplatin; MD=doctor.
Reproduced with permission from AP Venook, MD.
At the time of the inception of the trial in 2004, chemotherapy plus bevacizumab was the standard first-line therapy for CRC, with cetuximab showing efficacy in second- and third-line treatment. The combination of bevacizumab plus cetuximab was studied in refractory settings and found to be active. While the trial was open and enrolling patients, potential harm of combination biologics in the first-line setting became apparent, and the association of KRAS mutations with resistance to endothelial growth factor receptor-targeted drugs such as cetuximab was established. The trial design was consequently amended to discontinue the treatment arm combining bevacizumab and cetuximab with chemotherapy and to limit eligibility to patients who had tumors that were wild type in KRAS codons 12 and 13.
Key eligibility criteria for the trial included untreated mCRC or recurrence following adjuvant therapy received more than 12 months previously, tumor KRAS wild-type codons 12 and 13, Eastern Cooperative Oncology Group performance status of 0 to 1, and preserved organ function. The primary end point was overall survival (OS) of the intent-to-treat population. Secondary end points included progression-free survival (PFS) and evidence of interaction between chemotherapy and biological agents. Baseline characteristics were similar between the 1137 patients randomly assigned to chemotherapy plus cetuximab (n=578) and to chemotherapy plus bevacizumab (n=559; Table 1).
Baseline Characteristics
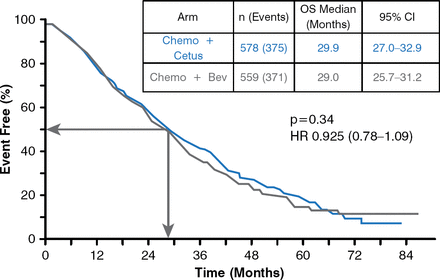
The primary end point of OS was not appreciably different between the study arms in the intention-to-treat population, with a median of 29.0 for bevacizumab and 29.9 for cetuximab (HR, 0.925; 95% CI, 0.78–1.09; p=0.34; Figure 2). In the subgroup of patients who received FOLFOX, median OS was 26.9 months for bevacizumab versus 30.1 months for cetuximab (HR, 0.9; 95% CI, 0.7–1.0; p=0.09).
Overall Survival
Bev=bevacizumab; Cetux=cetuximab; Chemo=chemotherapy; OS=overall survival.
Reproduced with permission from AP Venook, MD.
Median overall PFS was also similar between study arms: 10.4 months (95% CI, 9.6–11.3) and 10.8 months (95% CI, 9.7–11.4) in the cetuximab and bevacizumab arms, respectively (HR, 1.04; 95% CI, 0.91–1.17; p=0.55).
Grades 3 and 4 toxicities were numerically similar between the study arms. Grade 3 rash occurred exclusively in the cetuximab arm (7%). Grade 3 diarrhea was more prevalent in the cetuximab arm (n=59; 11%) than in the bevacizumab arm (n=45; 8%). Hypertension was more prevalent in the bevacizumab arm (n=35; 7%) than in the cetuximab arm (n=3; 1%). The reasons for discontinuation of treatment were similar in the study arms and consisted predominantly of disease progression and adverse events, study withdrawal, and change in therapy.
The findings support the use of FOLFIRI or FOLFOX chemotherapy combined with either bevacizumab or cetuximab as the first-line treatment in patients with KRAS wild-type mCRC. The OS exceeding 29 months resulting from either treatment establishes a new prognostic benchmark. While additional data are still being analyzed, the take-home message, according to Dr. Venook, is that patients have treatment choices, with first-line therapy reflecting their preference or concern about potential side effects.
- © 2014 MD Conference Express®