Summary
The Finland Herceptin [FinHer] trial results and concerns about cardiac toxicity with trastuzumab provided the rationale for the Trastuzumab for 6 Months or 1 Year in Treating Women with Nonmetastatic Breast Cancer That Can Be Removed by Surgery [PHARE; NCT00381901] trial. The primary objective of this noninferiority trial was to compare the effect of 6 versus 12 months of adjuvant treatment with trastuzumab on disease-free survival in patients with HER2-positive early breast cancer.
- Adjuvant/Neoadjuvant Therapy
- Oncology Clinical Trials
- Breast Cancer
One year of adjuvant treatment with trastuzumab improves survival in patients with human epidermal growth factor 2 (HER2)-positive early breast cancer [Piccart-Gebhart MJ et al. N Engl J Med 2005; Romond EH et al. N Engl J Med 2005]. The Finland Herceptin [FinHer] trial showed that patients treated with 9 weeks of adjuvant trastuzumab also had a similar survival benefit [Joensuu H et al. N Engl J Med 2006]. The FinHer results and concerns about cardiac toxicity with trastuzumab provided the rationale for the Trastuzumab for 6 Months or 1 Year in Treating Women with Nonmetastatic Breast Cancer That Can Be Removed by Surgery [PHARE; NCT00381901] trial presented by Xavier Pivot, MD, Institut National du Cancer, Boulogne-Billancourt, France. The primary objective of this noninferiority trial was to compare the effect of 6 versus 12 months of adjuvant treatment with trastuzumab on disease-free survival (DFS) in patients with HER2-positive early breast cancer.
A total of 3384 patients undergoing treatment with trastuzumab were randomized to continue treatment for 12 months (n=1690) or to stop treatment at 6 months (n=1690). The patients were stratified according to estrogen receptor status, and concurrent or sequential chemotherapy. Patients were evaluated with a clinical exam and left ventricular ejection fraction (LVEF) every 3 months for up to 24 months, and at 30 months. Mammography was performed every 6 months for up to 60 months. The primary endpoint was DFS; secondary endpoints were overall survival (OS) and cardiac toxicity.
Baseline patient characteristics were well balanced between the 2 study groups. About 58% of patients in both groups received concomitant chemotherapy, while about 42% received sequential chemotherapy. The mean duration of trastuzumab therapy was 11.8 months in the 12-month group and 6.3 months in the 6-month group.
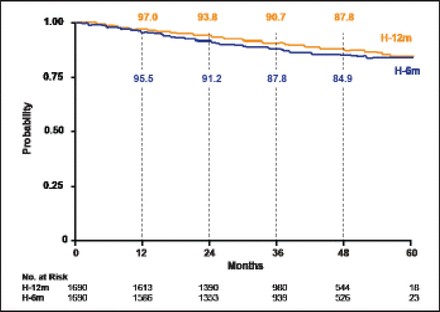
At median follow-up of 42.5 months, DFS events occurred in 10.4% (176 events) of patients treated for 12 months versus 13.0% (219 events) of those treated for 6 months (HR, 1.28; 95% CI, 1.05 to 1.56; p=0.29; Figure 1). The DFS events in the 12-month versus the 6-month treatment group included local recurrence (1.1% vs 1.4%), regional recurrence (0.6% vs 0.5%), and distant recurrence (6.4% vs 8.3%). Contralateral breast cancer occurred in 0.4% of the 12-month treatment group and 0.7% of the 6-month treatment group, and a second primary malignancy was reported in 1.5% of patients in each group. There were 66 deaths in the 12-month group versus 93 deaths in the 6-month group (HR, 1.47; 95% CI, 1.07 to 2.02; no p value reported).
Disease-Free Survival.
Reproduced with permission from X Pivot, MD.
The cardiac event (composite of clinical and LVEF findings) rate was 5.7% in the 12-month group versus 1.9% in the 6-month group (p<0.0001). LVEF was <50% in 6.3% of the 12-month group versus 4.7% in the 6-month group (p=0.04); it was <50% and decreased by >10% in 4.8% of the 12-month group versus 3.6% of the 6-month group (p=0.071); it was >50% and decreased by >15% in 7.4% of the 12-month group versus 7.0% of the 6-month group (not significant).
The results of the PHARE trial were inconclusive for the noninferiority hypothesis. Nevertheless, a trend favoring the standard 12 months of treatment in DFS and OS was observed.
- © 2012 MD Conference Express®