Summary
Results from a Phase 2 randomized controlled trial of the novel cholesteryl ester transfer protein (CETP) inhibitor evacetrapib are discussed. Compared with placebo or statin monotherapy, evacetrapib with or without a statin increased high-density lipoprotein cholesterol (HDL-C) and decreased low-density lipoprotein cholesterol (LDL-C) levels in patients with dyslipidemia [Effects of the CETP Inhibitor Evacetrapib Administered as Monotherapy or with Statins on HDL and LDL Cholesterol Trial; NCT01105975].
- Cardiology Clinical Trials
- Lipid Disorders
Stephen J. Nicholls, MBBS, PhD, Cleveland Clinic Heart & Vascular Institute, Cleveland, Ohio, USA, presented results from a Phase 2 randomized controlled trial of the novel cholesteryl ester transfer protein (CETP) inhibitor evacetrapib. Compared with placebo or statin monotherapy, evacetrapib with or without a statin increased high-density lipoprotein cholesterol (HDL-C) and decreased low-density lipoprotein cholesterol (LDL-C) levels in patients with dyslipidemia [Effects of the CETP Inhibitor Evacetrapib Administered as Monotherapy or with Statins on HDL and LDL Cholesterol Trial; NCT01105975].
Several CETP inhibitors are currently undergoing clinical evaluation. However, their effects in combination with the most commonly used statins have not been fully characterized. The purpose of this randomized, double-blind, multicenter, dose-ranging study was to examine the biochemical effects, safety, and tolerability of evacetrapib as monotherapy and in combination with statins in patients with hypercholesterolemia or low HDL-C levels. The co-primary endpoints were percentage changes from baseline in HDL-C and LDL-C after 12 weeks of treatment. Following a dietary lead-in, 398 patients were randomly assigned to one of 10 treatment groups for 12 weeks: placebo; evacetrapib monotherapy (30, 100, or 500 mg/day); or statin therapy (simvastatin, 40 mg/day; atorvastatin, 20 mg/day; or rosuvastatin, 10 mg/day) with or without evacetrapib 100 mg/day. A total of 393 patients received the study drug and were included in the final analysis.
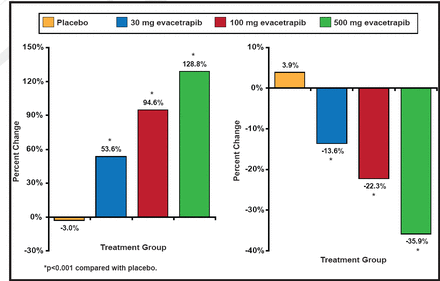
The mean baseline HDL-C level was 55.1 (SD, 15.3) mg/dL [1.42 mmol/L] and the mean baseline LDL-C level was 144.3 (SD, 26.6) mg/dL [3.73 mmol/L]. As monotherapy, evacetrapib produced dose-dependent increases of HDL-C of 30.0 to 66.0 mg/dL [0.78 to 1.71 mmol/L] (53.6% to 128.8%) compared to a decrease with placebo of −0.7 mg/dL [−0.02 mmol/L] (−3.0%; p<0.001 for all compared with placebo). Decreases in LDL-C were −20.5 to −51.4 mg/dL [−0.53 to 1.33 mmol/L] (−13.6% to −35.9%) compared to an increase with placebo of 7.2 mg/dL [0.19 mmol/L] (3.9%; p<0.001 for all compared with placebo; Figure 1).
Percent Changes in HDL-C and LDL-C.
Reproduced with permission from S. Nicholls, MBBS, PhD.
In combination with statin therapy, evacetrapib, 100 mg/day, produced absolute increases in HDL-C of 42.1 to 50.5 mg/dL [1.09 to 1.31 mmol/L] (78.5% to 88.5%; p<0.001 for all compared with statin monotherapy) and absolute decreases in LDL-C of −67.1 to −75.8 mg/dL [1.74 to 1.96 mmol/L] (−11.2% to −13.9%; p<0.001 for all compared with statin monotherapy). Compared with evacetrapib monotherapy, the combination of statins and evacetrapib resulted in greater reduction in LDL-C (p<0.001), but no greater increase in HDL-C (p=0.39). Evacetrapib was well tolerated, with a low rate of treatment-related adverse events or discontinuation of therapy. No evidence of adverse blood pressure or mineralocorticoid effects was observed as was seen previously with torcetrapib.
The development of CETP inhibitor drugs to increase HDL-C levels has been challenging and marked by failure with the first agent developed. In the ILLUMINATE trial, torcetrapib increased cardiovascular death and had off-target effects (increase in aldosterone) that led to increases in blood pressure [Barter PJ et al. N Engl J Med 2007]. However, the outcomes from this and other Phase 2 trials with anacetrapib and dalcetrapib suggest promise for second generation CETP inhibitors as cardioprotective agents.
Two large cardiovascular outcome studies (dal-OUTCOMES [Schwartz GG et al. Am Heart J 2009] and REVEAL HPS-3 TIMI-55 [Melloni C et al. Am Heart J 2010]) are ongoing to determine whether CETP inhibitors can further reduce the substantial residual risk of cardiovascular disease still observed in patients with established coronary artery disease despite the use of existing lipid therapies.
- © 2011 MD Conference Express®