Summary
Atrial fibrillation (AF) becomes more common with increasing age. Data from an unpublished new analysis of the Global Study to Assess the Safety and Effectiveness of Edoxaban (DU-176b) vs Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation [ENGAGE AF-TIMI 48] support edoxaban as an alternative treatment to warfarin in elderly patients who require anticoagulation therapy. This article discusses a prespecified analysis that evaluated the association of age on study outcomes and the efficacy and safety of edoxaban relative to warfarin in the elderly with AF.
- ENGAGE-AF TIMI-48
- arrhythmias
- cardiology clinical trials
Atrial fibrillation (AF) becomes more common with increasing age. Elderly patients are at greater risk of stroke while having a higher bleeding risk with anticoagulation. Data from an unpublished new analysis of the Global Study to Assess the Safety and Effectiveness of Edoxaban (DU-176b) vs Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation [ENGAGE AF-TIMI 48; Giugliano RP et al. N Engl J Med. 2013] support edoxaban as an alternative treatment to warfarin in elderly patients who require anticoagulation therapy, stated Eri Toda Kato, MD, PhD, TIMI Study Group, Boston, Massachusetts, USA. She presented a prespecified analysis that evaluated the association of age on study outcomes and the efficacy and safety of edoxaban relative to warfarin in the elderly with AF.
The ENGAGE AF-TIMI 48 trial [Giugliano RP et al. N Engl J Med. 2013] established the noninferiority of high-dose edoxaban (HDE; 60 mg) and low-dose edoxaban (LDE; 30 mg) to warfarin for the primary outcome of stroke and systemic embolic event (SEE) in 21 105 patients with nonvalvular AF and a CHADS2 score ≥ 2. The edoxaban dose was halved for patients having a creatinine clearance between 30 and 50 mL/min or a body weight ≤ 60 kg or for those taking potent P-glycoprotein inhibitors.
Their key characteristics in the prespecified age categories for the new analyses are detailed in Table 1.
Patient Characteristics by Age in ENGAGE AF-TIMi 48
The association of age on outcomes was determined by examining the event rate in the warfarin group, which eliminated the possible influence of the edoxaban dose adjustment. This analysis revealed a significant linear association between age and stroke/SEE, ischemic stroke (IS), International Society of Thrombosis and Haemostasis (ISTH) major bleeding criteria, and intracranial hemorrhage (ICH; P < .001 for all). In the ≥ 75 and < 65 age groups, the absolute risk difference was 4% for stroke/SEE and 8% for ISTH at 3 years (P < .001 for both), underscoring the strong relation between age and risk, which was amplified for bleeding.
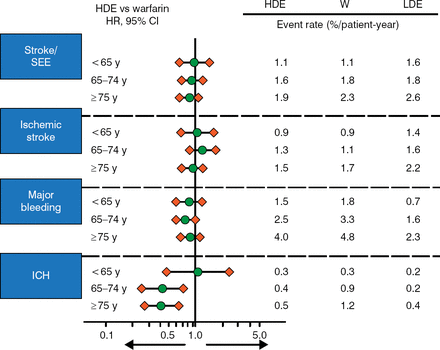
The risk of events was increased with age for all outcomes and all treatments (Figure 1). HDE provided a greater reduction in ICH in patients aged > 65 years, while HDE and warfarin had a similar effect on stroke, SEE, IS, major bleeding, and ICH in most other groups. LDE reduced major bleeding and ICH but increased this risk of stroke/SEE and IS. There was no evidence that age modified the effectiveness of treatment with edoxaban on stroke/SEE or any other outcome (P Interaction > .05).
Risk of Events by Age and Treatment in ENGAGE AF-TIMI 48
ENAGE AF-TIMI 48, Global Study to Assess the Safety and Effectiveness of Edoxaban (DU-176b) vs Standard Practice of Dosing With Warfarin in Patients With Atrial Fibrillation; HDE, high-dose edoxaban; ICH, intracranial hemorrhage; LDE, low-dose edoxaban; SEE, systemic embolic event; W, warfarin.
Reproduced with permission from ET Kato, MD, PhD.
In the efficacy analysis of edoxaban vs warfarin, the absolute risk reduction (ARR) showed that HDE reduced stroke/SEE in all age groups but was more pronounced in patients aged ≥ 75 years (−40 events per 10000 patient-years vs −6 and −20 for the < 65 and 65–74 age groups). It also reduced ICH in patients aged ≥ 75 years but not the other age groups (−18 events per 10 000 patient-years vs +2 and +17 for the < 65 and 65–74 age groups). LDE did not reduce stroke/SEE or IS.
The safety analysis showed a dramatic ARR with HDE and LDE for ISTH major bleeding in all age groups and for ICH in patients aged > 65 years (Table 2).
Safety Analysis: Absolute Risk Reduction With Edoxaban vs Warfarina
The prespecified primary net clinical outcome (approved by the Food and Drug Administration when designing the trial) comprising stroke/SEE, major bleeding, or all-cause death was reduced with HDE and LDE in all age groups, with a more marked ARR in the ≥ 75 age group (−144 and −251, respectively, vs −5 and −35 for the < 65 age group and −93 and −90 for the 65–74 age group).
In conclusion, this analysis from ENGAGE-TIMI 48 showed that increasing age was associated with the risk of stroke/SEE, IS, ISTH major bleeding, and ICH and that the increased risk for ISTH major bleeding and ICH were more marked. Age did not affect outcomes with edoxaban vs warfarin. Edoxaban reduced the absolute risk of ISTH major bleeding and ICH and provided a superior net clinical benefit vs warfarin by decreasing the bleeding risk, particularly in patients aged ≥ 75 years. The absolute benefits with edoxaban vs warfarin were greater in patients aged ≥ 75 years because of their higher risk for stroke and bleeding.
- © 2014 MD Conference Express®