Summary
Hyperglycemia is a common condition among cardiac surgery patients, and it occurs in approximately half of all patients following surgery. Although it is agreed that controlling hyperglycemia reduces risk of organ failure, infection, and mortality, the ideal target range for blood glucose in the perioperative period remains unknown. This article discusses the GLUCO-CABG trial [NCT01792830], which examined if intensive glucose control while the patient is in the ICU following coronary artery bypass grafting can improve outcomes.
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Interventional Techniques & Devices
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Endocrinology
- Diabetes & Metabolic Syndrome
- Interventional Techniques & Devices
Hyperglycemia is a common condition among cardiac surgery patients, and it occurs in approximately half of all patients following surgery. Although it is agreed that controlling hyperglycemia reduces risk of organ failure, infection, and mortality, the ideal target range for blood glucose (BG) in the perioperative period remains unknown. The 2009 American Diabetes Association (ADA) guidelines recommended a range of 140 to 180 mg/dL BG in intensive care unit (ICU) patients [Moghissi ES et al. Endocr Pract. 2009], but the intensive glucose control required to deliver this range results in severe hypoglycemia (< 40 mg/dL) in 5% to 20% of ICU patients [Umpierrez et al. J Clin Endocrinol Metabol. 2002].
Guillermo Umpierrez, MD, Emory University School of Medicine, Atlanta, Georgia, USA, presented results from the GLUCO-CABG trial [NCT01792830]. The objective of this randomized, controlled study was to determine if intensive glucose control while the patient is in the ICU following coronary artery bypass grafting (CABG) can improve outcomes. The primary end point was a composite end point that included a variety of potential surgical complications. The study included men and women between 18 and 80 years, with or without a history of diabetes, who had undergone CABG with or without valve surgery, and had displayed hyperglycemia (defined as BG> 140 mg/dL) either during surgery or during their stay in the ICU. At baseline, patient characteristics were similar between the 2 treatment groups (Table 1).
Patient Characteristics at Baseline
Patients were randomized to either intensive control of BG (range, 100 to 140 mg/dL) using computer algorithm to guide the infusion of insulin or conservative therapy within the ADA-recommended range (141 to 180 mg/dL). After attrition, a total of 148 patients in each group achieved 80% power for the study to detect an odds ratio of 0.35 in composite outcome (α = 0.05).
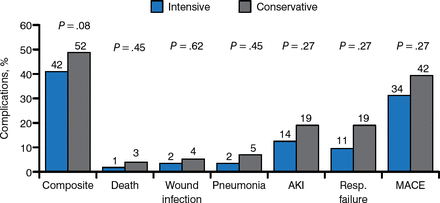
In the 90 days following hospital discharge, patients treated with intensive glucose control in the ICU had a 42% rate of surgical complications, compared with 52% in patients treated with conservative control (Figure 1). The difference between the 2 groups was not significant, however, on a composite of complications that included death, pneumonia, acute kidney injury (AKI), respiratory failure, wound infections, or major cardiovascular events (MACEs) (P = .08).
Comparison of Intensive and Conservative Glucose Control on Perioperative Complications
AKI, acute kidney injury; Resp., respiratory; MACE, major cardiovascular events.
Reproduced with permission from G Umpierrez, MD.
Composite of complications: death, wound infection, pneumonia, AKI, respiratory failure, and MACEs. Low rates of hypoglycemia were achieved using the computer algorithm to guide insulin infusion, and no patients had a BG < 40 mg/dL (2.2 mmol/L).
In summary, this study found that intensive glucose control in patients with hyperglycemia undergoing CABG surgery that targeted a BG of 100 to 140 mg/dL during the perioperative period did not significantly reduce complications or mortality compared with a less strict target of 141 to 180 mg/dL.
- © 2014 MD Conference Express®