Summary
Patients with type 2 diabetes mellitus (T2DM) require long-term treatment with a hypoglycemic agent, but these agents can be difficult in terms of patient adherence. This article presents 6-year follow-up data on the efficacy of a once-weekly formulation of exenatide. The phase 3 Effects of Exenatide Long-Acting Release on Glucose Control and Safety in Subjects With Type 2 Diabetes Mellitus [DURATION-1; NCT00308139] is the longest assessment of a glucagon-like peptide–1 receptor agonist reported to date.
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
- Diabetes & Endocrinology Clinical Trials
- Endocrinology
- Diabetes & Metabolic Syndrome
- Diabetes Mellitus
- Hyperglycemia/Hypoglycemia
Patients with type 2 diabetes mellitus (T2DM) require long-term treatment with a hypoglycemic agent, but these agents can be difficult in terms of patient adherence. Eric J. Klein, MD, Capital Clinical Research Center, Olympia, Washington, USA, presented 6-year follow-up data on the efficacy of a once-weekly formulation of exenatide. The phase 3 Effects of Exenatide Long-Acting Release on Glucose Control and Safety in Subjects With Type 2 Diabetes Mellitus [DURATION-1; NCT00308139] is the longest assessment of a glucagon-like peptide–1 receptor agonist reported to date [MacConell L et al. Diabetes Metab Syndr Obes. 2013].
Inclusion criteria for DURATION-1 included T2DM (treated with either diet and exercise or oral antidiabetic agents), HbA1c 7.1% to 11.0%, fasting plasma glucose (FPG) < 16 mmol/L, and body mass index 25 to 45 kg/m2 [Taylor L et al. BMC Endocr. 2011; Buse JB et al. Diabetes Care. 2010; Drucker DJ et al. Lancet. 2008]. Patients were randomly assigned 1:1 to weekly or twice-daily exenatide. At the end of 30 weeks, patients could continue on the extension trial with weekly exenatide. According to Dr Klein, there were no differences in the baseline characteristics between the intention-to-treat (ITT) population and the 6-year completer population.
Efficacy data were reported for the population that completed 6 years of follow-up (n = 127). Safety data reported for the ITT population (n = 295) included all individuals who received ≥ 1 dose of exenatide. Drug discontinuation over 6 years of follow-up occurred in 59% of the ITT population (n = 175).
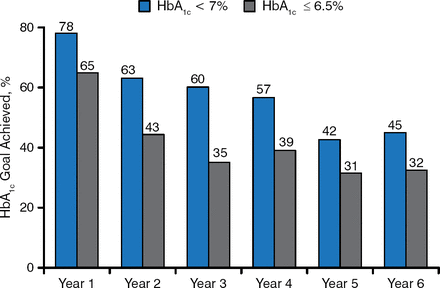
At year 1, the HbA1c level had dropped by 2.2%. The HbA1c level increased over the next 5 years, but at the end of the study the least squares (LS) mean change was −1.6%. The HbA1c level over the entire 6 years was significantly lower than baseline (P < .05). The percentages of patients whose HbA1c levels were < 7% or ≤ 6.5% during the course of the study are shown in Figure 1.
Patients Whose HbA1c Levels Were < 7% or ≤ 6.5% Over Time
Six-year completer population (n = 127).
Reproduced with permission from EJ Klein, MD.
FGP decreased by 2.7 mmol/L in the first year. Over the next 5 years, FPG increased but remained lower at study end (−1.6 mmol/L). FPG over the entire 6 years was significantly lower than at baseline (P < .05). With regard to weight, patients lost 4.7 kg the first year, regained some weight over the next 2 years, and lost weight again in years 4, 5, and 6. At year 6, the LS mean weight loss was 4.3 kg. Differences from baseline were significantly lower at each year of follow-up except for year 4. There was a decrease in blood pressure after the first year of treatment, but this difference was not sustained over the course of the study.
There were no incremental safety findings, including occurrences of major hypoglycemia, observed with long-term treatment. The most common adverse events (AEs) reported included upper respiratory tract infection (43%), nasopharyngitis (29%), diarrhea (27%), sinusitis (24%), and arthralgia (21%). Nausea (mostly mild) and injection-site reactions were the most common AEs with exenatide once weekly during the first 30 weeks, but these were not the most common AEs in the 6 years of follow-up.
Treatment-emergent AEs leading to withdrawal from week 30 to 6 years were infrequent (6.6%); 107 serious AEs were reported in 61 patients from week 30 to year 6. AEs of special interest are listed in Table 1.
AEs of Special Interest
In conclusion, long-term therapy with once-weekly exenatide is feasible and well tolerated. This treatment regimen resulted in sustained improvements in glycemic control and weight over 6 years in the 40% of enrollees who continued therapy.
- © 2014 MD Conference Express®