Summary
This article discusses the results from the Assessment of Weekly Administration of LY2189265 in Diabetes-4 trial [AWARD-4; NCT01191268], which indicate that dulaglutide, in combination with insulin lispro, is an effective and safe option for treatment intensification in type 2 diabetes patients who are inadequately controlled on 1 or 2 doses of insulin.
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Diabetes & Endocrinology Clinical Trials
- Hyperglycemia/Hypoglycemia
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
Results from the Assessment of Weekly Administration of LY2189265 in Diabetes-4 trial [AWARD-4; NCT01191268], presented by Johan Jendle, MD, PhD, Endocrine and Diabetes Center, Karlstad and Faculty of Health Sciences and Medicine, Orebro University, Sweden, indicate that dulaglutide, in combination with insulin lispro, is an effective and safe option for treatment intensification in type 2 diabetes (T2D) patients who are inadequately controlled on 1 or 2 doses of insulin.
A basal-bolus insulin regimen is often recommended for patients with T2D who are unable to achieve target glycemic control with conventional therapy. However, many of these patients fail to achieve optimal HbA1c levels, possibly because of the increased frequency of hypoglycemia and weight gain associated with this regimen. The combination of insulin and a glucagon-like peptide–1 (GLP-1) receptor agonist is being examined as an alternative regimen, but studies to date have included only basal insulin. AWARD-4 is the first randomized trial to explore the use of a GLP-1 receptor agonist with prandial insulin. The objective of this 52-week, parallel-arm, open-label, phase 3 study was to compare dulaglutide with basal glargine when used in combination with prandial insulin (lispro). Insulin glargine and insulin lispro were titrated in an attempt to reach glycemic targets.
The study enrolled patients with T2D who were inadequately controlled, with HbA1c levels ≥ 7% and ≤ 11%. In addition, patients also were taking 1 or 2 stable insulin doses daily for 3 months and had body mass indexes (BMIs) ≥ 23 and ≤ 45 kg/m2. Subjects were randomized (1:1:1) to once weekly dulaglutide 1.5 mg, once weekly dulaglutide 0.75 mg, or once daily glargine. All participants also received insulin lispro 3 times daily with meals. Both glargine and insulin lispro were titrated to target on the basis of the previous stable insulin dose. The primary objective was to assess the noninferiority of dulaglutide 1.5 mg to glargine on HbA1c from baseline to week 26 using a 0.4% margin. If noninferiority was met, then the superiority of dulaglutide 1.5 mg and the noninferiority and superiority of dulaglutide 0.75 mg were tested.
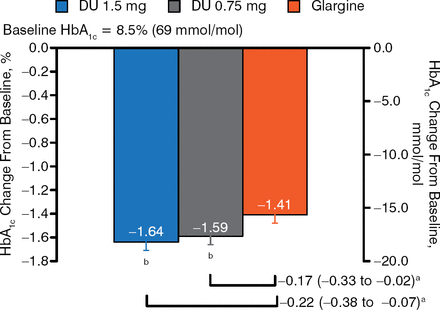
Participants had a mean age of 59 years, a mean duration of disease of 13 years, a mean HbA1c level of 8.5%, and a mean BMI of 32 kg/m2. The majority (> 75%) were on metformin prior to randomization and/or basal insulin only. The mean total daily insulin dose was 56 U. At week 26, patients treated with dulaglutide doses had greater reductions in HbA1c compared with those receiving glargine (Figure 1). This difference was maintained at 52 weeks.
HbA1c Change From Baseline: 26 Weeks
aTreatment difference (nominal 95% CI).
b P < .025, superiority vs glargine (1-sided, adjusted to control for type I error).
Reproduced with permission from J Jendle, MD.
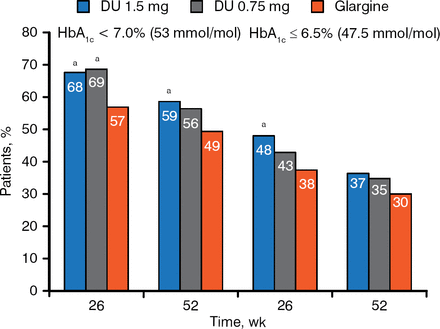
At both weeks 26 and 52, a higher percentage of patients treated with either dose of dulaglutide reached HbA1c target compared with patients treated with glargine (Figure 2).
HbA1c Targets at 26 and 52 Weeks
a P < .05 vs glargine.
Reproduced with permission from J Jendle, MD.
Patients treated with glargine gained weight, whereas dulaglutide 1.5 mg was weight neutral over time. The weight difference between the dulaglutide 1.5-mg dose and glargine at week 52 was 3.3 kg (P < .001). The incidence of hypoglycemia was significantly lower with dulaglutide 1.5 mg compared with glargine at week 26 (Table 1).
Number of Hypoglycemic Events at 26 Weeks
There were no differences in overall adverse events. As expected, there were significantly (P < .001) more reports of gastrointestinal events (nausea, diarrhea, and vomiting) among patients treated with dulaglutide compared with glargine. Reports of severe hypoglycemia were low for all treatment groups, as were injection site reactions. There were no reports of pancreatic cancer.
- © 2014 MD Conference Express®