Summary
The 2012 American Diabetes Association/European Association for the Study of Diabetes position statement for the management of hyperglycemia recommends, as 1 of the 3-drug combinations, the addition of glucagon-like peptide–1 receptor agonists to basal insulin analogs (or conversely, insulin to GLP-1 receptor agonists) after initial therapy with metformin. This article presents the results of the Effect of Liraglutide Versus Placebo When Added to Basal Insulin Analogues With or Without Metformin in Subjects With Type 2 Diabetes trial [LIRA-ADD2BASAL; NCT01617434], comparing the efficacy and safety of liraglutide versus placebo when added to basal insulin analogs.
- Hyperglycemia/Hypoglycemia
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
- Endocrinology
- Diabetes & Metabolic Syndrome
- Hyperglycemia/Hypoglycemia
- Diabetes & Endocrinology Clinical Trials
- Diabetes Mellitus
Jorma Lahtela, MD, PhD, Tampere University Hospital, Tampere, Finland, presented the results of the randomized, placebo-controlled Effect of Liraglutide Versus Placebo When Added to Basal Insulin Analogues With or Without Metformin in Subjects With Type 2 Diabetes trial [LIRA-ADD2BASAL; NCT01617434; Lahtela J et al. EASD 2014 (Oral Presentation 37)], comparing the efficacy and safety of liraglutide versus placebo when added to basal insulin analogs (insulin detemir or insulin glargine) in patients with type 2 diabetes mellitus (T2DM). Liraglutide 1.8 mg added to basal insulin analogs significantly improved glycemic control and reduced weight and blood pressure compared with placebo. Typical gastrointestinal symptoms and nonsevere hypoglycemia were reported more frequently with liraglutide than with placebo.
The 2012 American Diabetes Association/European Association for the Study of Diabetes position statement for the management of hyperglycemia recommends, as 1 of the 3-drug combinations, the addition of glucagon-like peptide–1 (GLP-1) receptor agonists to basal insulin analogs (or conversely, insulin to GLP-1 receptor agonists) after initial therapy with metformin [Inzucchi SE et al. Diabetes Care. 2012]. The goal of this trial was to establish the superior efficacy and acceptable safety of liraglutide, compared with placebo, when added to preexisting basal insulin analog with or without metformin in patients with inadequately controlled T2DM.
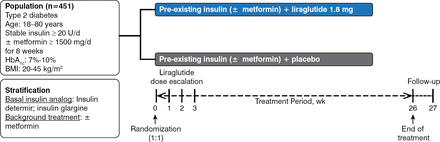
Patients were randomized 1:1 to receive once daily liraglutide 1.8 mg or placebo added to preexisting treatment for 26 weeks in this multicenter, multinational, double-blind, parallel-group design study (Figure 1). Patients with T2DM, aged 18 to 80 years, with body mass indexes (BMIs) of 20 to 45 kg/m2 and HbA1c level of 7% to 10%, and on stable insulin analog dose ≥ 20 U/d with or without stable metformin ≥ 1500 mg/d were eligible for participation. Insulin adjustments above the pretrial dose were not allowed.
Study Design of LIRA-ADD2BASAL
BMI, body mass index.
Reproduced with permission from J Lahtela, MD.
A total of 450 patients were randomized in the trial, with a mean duration of diabetes of 12.1 years, a mean BMI of 32 kg/m2, and a geometric mean pretrial insulin dose of 40.5 U. The mean baseline HbA1c level was similar between groups (8.2% liraglutide, 8.3% placebo).
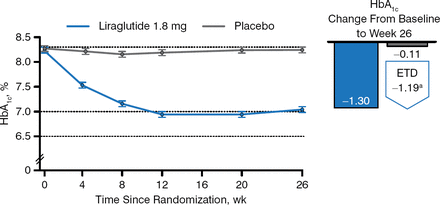
Three hundred sixty-five patients completed the trial. The primary end point was the change in HbA1c from baseline to week 26. Patients taking liraglutide had a greater decrease in HbA1c from baseline than those taking placebo (−1.3 and −0.11, respectively; Figure 2), and more liraglutide recipients reached HbA1c < 7.0% (59.2% vs 14.0%) and HbA1c ≤ 6.5% (42.9% vs 3.6%) (P < .0001 for both) despite using a lower mean estimated daily dose of basal insulin analog compared with placebo (35.8 vs 40.0 U).
Change in HbA1c in Patients Taking Liraglutide Versus Placebo Added to Basal Insulin Analogs
Estimated means ± standard errors, from mixed model for repeated measurements. ETD, estimated treatment difference.
Reproduced with permission from J Lahtela, MD.
a95% CI, −1.39 to −0.99 (P < .0001).
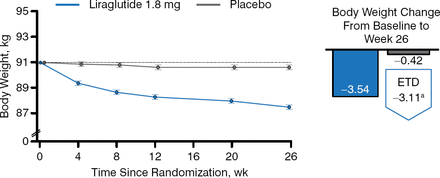
Patients taking liraglutide also achieved greater decreases from baseline in fasting plasma glucose (FPG; −26 and −3 mg/dL, respectively), incremental postprandial self-measured plasma glucose (−17 and −7 mg/dL, respectively), body weight (Figure 3), systolic blood pressure (SBP) (−6 and −1 mm Hg, respectively), and lipids.
Change in Weight in Patients Taking Liraglutide Versus Placebo Added to Basal Insulin Analogs
Estimated mean change from baseline to week 26, from mixed model for repeated measurements. ETD, estimated treatment difference.
Reproduced with permission from J. Lahtela, MD.
a95% CI, −3.85 to −2.37 (P < .0001).
Nausea and vomiting occurred more frequently with liraglutide than placebo (22% vs 3% and 9% vs 1%, respectively). Minor hypoglycemia (plasma glucose < 56 mg/dL) occurred in 18% and 12% of liraglutide and placebo recipients, respectively. No severe hypoglycemic events (requiring assistance of another person) were reported during this trial.
In summary, the addition of liraglutide to insulin detemir or insulin glargine with or without metformin significantly improved glycemic control, which was attributed to the effect of liraglutide on both FPG and postprandial glucose levels. Additionally, liraglutide induced greater weight loss and reductions in SBP and selected lipids compared with placebo. Adverse effects were similar to those seen in other trials of liraglutide.
- © 2014 MD Conference Express®