Summary
This article reports on results of the Effect of Liraglutide on Body Weight in Non-diabetic Obese Subjects or Overweight Subjects With Co-morbidities study [SCALE; NCT01272219], a 56-week randomized, double-blind, placebo-controlled trial demonstrating the efficacy of liraglutide in inducing and maintaining weight loss, and enhancing health-related quality of life, in 3751 obese or overweight patients with or without prediabetes.
- Nutrition Clinical Trials
- Obesity
- Diabetes & Endocrinology Clinical Trials
- Endocrinology
- Diabetes & Metabolic Syndrome
- Nutrition Clinical Trials
- Obesity
- Diabetes & Endocrinology Clinical Trials
Ken Fujioka, MD, Scripps Clinic, La Jolla, California, USA, reported results of the Effect of Liraglutide on Body Weight in Non-diabetic Obese Subjects or Overweight Subjects With Co-morbidities study [SCALE; NCT01272219], a 56-week randomized, double-blind, placebo-controlled trial demonstrating the efficacy of liraglutide in inducing and maintaining weight loss, and enhancing health-related quality of life (HRQOL), in 3751 obese or overweight patients with or without prediabetes.
The diminished QOL attributable to obesity can be improved by weight loss [Warkenten LM et al. Obesity Res. 2014; Ul Haq et al. Obesity. 2013]. Liraglutide is a long-acting glucagon-like peptide-1 agonist approved by the Food and Drug Administration for the treatment of type 2 diabetes, and it also decreases appetite and helps maintain a lower body weight. The primary objective of SCALE was to examine the efficacy of liraglutide (3.0 mg) as compared with placebo on weight loss throughout 56 weeks. Secondary objectives included patient-reported HRQOL outcomes of health status, impact of weight on QOL, and effect of prescription anti-obesity medication.
Inclusion criteria were body mass index (BMI) ≥ 30 kg/m2 or ≥ 27 kg/m2 in those with comorbidities, stable body weight, and the prior failure of a weight loss diet.
Patients with or without prediabetes were randomized 2:1 to liraglutide 3.0 mg/day (n = 2487; achieved in a 4-week period of dose escalation) or placebo (n = 1244). After 52 weeks, those without prediabetes were randomized 1:1 to continued liraglutide therapy or placebo for another 18 weeks. Those with prediabetes continued the original randomized treatment through 172 weeks. The present data are from the first 52 weeks of the study, which was the period of identical treatment for those with and without prediabetes.
Patients receiving liraglutide and placebo were comparable at baseline in terms of mean age, gender, mean body weight, mean ranges of BMI defining overweight and categories of obesity, race or ethnicity, prevalence of hypertension and/or dyslipidemia, prior cardiovascular disease, and diagnosis of prediabetes.
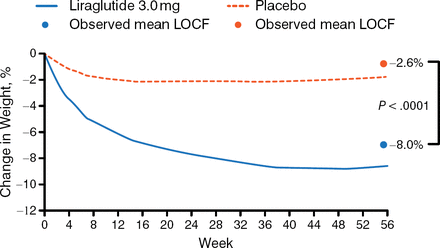
The mean weight loss from baseline to week 56 was significantly greater in those receiving liraglutide than in the placebo group (−8.0% vs −2.6%; P < .0001; Figure 1).
Mean Weight Loss
LOCF, last observation carried forward.
Reproduced with permission from K Fujioka, MD.
In addition, liraglutide led to significant improvements in overall physical health and function, general health, body pain, overall mental health, social functioning, mental health, vitality, self-esteem, sexuality, and work performance. Overall, the odds ratio of a better outcome for liraglutide treatment vs placebo was 1.6 (95% CI, 1.4 to 1.9; P < .0001).
Other patient-reported outcomes of the influence of liraglutide treatment indicated that the medication was associated with improved weight management but also had side effects. Adverse effects that occurred in ≥ 5% of patients included nausea (which persisted throughout the 56 weeks), diarrhea, constipation, vomiting, loss of appetite, and dyspepsia. A variety of other adverse effects were seemingly not related to liraglutide use because they occurred with comparable frequencies in both groups (Table 1).
Adverse Events Occurring in ≥ 5% of Patients
Despite the inconveniences of the drug-related side effects, the researchers concluded that liraglutide 3.0 mg used as an adjunct to diet and exercise is a means of achieving significant weight loss, and confers patient-related improvements in physical and mental health.
- © 2014 MD Conference Express®