Summary
Apremilast, an oral phosphodiesterase 4 inhibitor that works intracellularly to regulate inflammatory mediators, has been tested in the Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) program, which consists of 2 trials: ESTEEM 1 [NCT01194219] and ESTEEM 2 [NCT01232283]. This article presents the 52-week results of the ESTEEM 2 trial.
- Skin Diseases
- Dermatology Clinical Trials
- Skin Diseases
- Dermatology
- Dermatology Clinical Trials
Apremilast, an oral phosphodiesterase 4 inhibitor that works intracellularly to regulate inflammatory mediators, has been tested in the Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis (ESTEEM) program, which consists of 2 trials: ESTEEM 1 [NCT01194219] and ESTEEM 2 [NCT01232283]. Carle Paul, MD, Toulouse University, Toulouse, France, presented the 52-week results of the ESTEEM 2 trial.
Patients with moderate-to-severe plaque psoriasis, defined as Psoriasis Area and Severity Index (PASI) ≥ 12, body surface area ≥ 10%, and Static Physician's Global Assessment (sPGA) ≥ 3, were randomized 2:1 to treatment with apremilast 30 mg BID (n = 274) or placebo (n = 137). At week 16, placebo patients switched to apremilast, and all patients received apremilast through week 32. At week 32, patients receiving apremilast from baseline who achieved PASI-50 response were rerandomized to continue apremilast or receive placebo. On loss of 50% of PASI improvement achieved at week 32, patients who had been rerandomized to placebo were switched back to apremilast. The primary end point was PASI-75 at week 16.
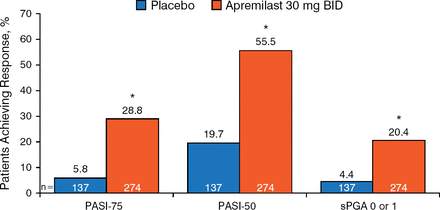
The included patients were aged ≥ 18 years and had moderate-to-severe chronic plaque psoriasis for ≥ 12 months. The full analysis set included 411 patients. At week 16, significantly more patients treated with apremilast achieved PASI-75 (28.8% vs 5.8%; P < .0001) when compared with placebo. Significant findings were also noted in PASI-50 (55.5% vs 19.7%; P < .0001) and sPGA 0 or 1 (20.4% vs 4.4%; P < .0001; Figure 1).
PASI-75, PASI-50, and sPGA Responses at Week 16
Data are the full analysis set, last observation carried forward (N = 411). PASI, Psoriasis Area and Severity Index; sPGA, Static Physician's Global Assessment.
*P < .0001 vs placebo; sPGA score of 0 (clear) or 1 (almost clear) with ≥2-point reduction from baseline.
Reproduced with permission from C Paul, MD.
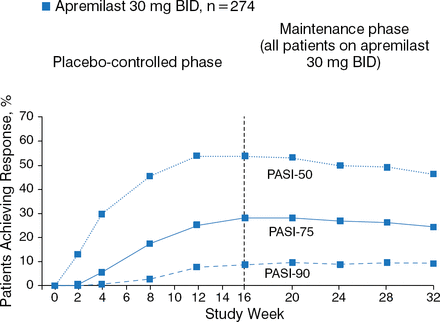
PASI responses generally were maintained through week 32 (Figure 2).
PASI-50, PASI-75, and PASI-90 Throughout 32 Weeks in Patients Receiving Apremilast From Baselinea
PASI, Psoriasis Area and Severity Index.
aData using nonresponder imputation at each time point.
Reproduced with permission from C Paul, MD.
Among patients randomized at baseline to apremilast, the mean percent change from baseline PASI at week 52 was −45.7% in week 32 PASI-50 nonresponders and −74.4% in week 32 PASI-50 responders. Among patients randomized at baseline to placebo and switched to apremilast at week 16, the mean percent change from baseline PASI at week 52 was −24.7% in week 32 PASI-50 nonresponders and −71.8% in week 32 PASI-50 responders.
The mean improvement in PASI generally remained stable from week 32 (77%) to 52 (74%) in patients with PASI-50 rerandomized to apremilast at week 32. The median time to loss of 50% of PASI improvement at week 32 was 12.4 weeks for patients rerandomized to placebo. Approximately 66% of patients rerandomized to placebo regained PASI-50 response after reinitiation of treatment with apremilast (duration of retreatment ranged from 2.6 to 18.3 weeks).
Most adverse events (AEs) were mild or moderate and did not lead to discontinuation. The serious AE rate was low and comparable among treatment arms. AEs occurring in ≥ 5% of patients exposed to apremilast from weeks 0 to 52 were nausea (16.6%), diarrhea (14.5%), nasopharyngitis (14.5%), upper respiratory tract infection (9.2%), tension headache (7.6%), vomiting (6.3%), headache (5.8%), and back pain (5.3%). Changes in laboratory parameters were transient with no trends observed.
Apremilast 30 mg BID significantly reduced (P < .0001) the severity of moderate-to-severe psoriasis throughout 16 weeks, with responses generally maintained to 52 weeks. The results of ESTEEM 2 confirm the efficacy of apremilast in patients with psoriasis, as reported in ESTEEM 1. Apremilast was generally well tolerated up to 52 weeks. Prof Paul concluded that apremilast represents a novel therapeutic option for patients with moderate-to-severe plaque psoriasis.
- © 2014 MD Conference Express®