Summary
Secretion of interleukin (IL)-23 by myeloid dendritic cells plays a role in the initiation of psoriasis. In addition, chronic psoriasis is maintained, at least in part, by the continued secretion of IL-23, which promotes the secretion of chemokines and antimicrobial peptides by keratinocytes, resulting in amplification that causes further IL-23 secretion. This article discusses the combination of IL-23 blockade with a single dose of the monoclonal antibody BI 655066 in the Single Rising Dose Study of BI 655066 in Patients With Moderate and Severe Psoriasis [NCT01577550].
- Skin Diseases
- Dermatology Clinical Trials
- Dermatology
- Skin Diseases
- Dermatology Clinical Trials
Interleukin (IL)-23 blockade with a single dose of the monoclonal antibody BI 655066 was safe and well tolerated in the Single Rising Dose Study of BI 655066 in Patients With Moderate and Severe Psoriasis [NCT01577550] presented by James Krueger, MD, PhD, Rockefeller University, New York, New York, USA.
Secretion of IL-23 by myeloid dendritic cells plays a role in the initiation of psoriasis [Lowes MA et al. Ann Rev Immunol. 2014]. In addition, chronic psoriasis is maintained, at least in part, by the continued secretion of IL-23, which promotes the secretion of chemokines and antimicrobial peptides by keratinocytes, resulting in amplification that causes further IL-23 secretion. BI 655066 is a monoclonal antibody that selectively targets the p19 subunit of IL-23 to prevent IL-23 activity. The purpose of this first-in-human study was to evaluate the safety and tolerability of BI 655066 in patients with moderate to severe plaque psoriasis.
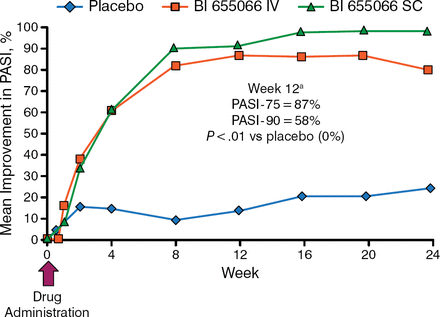
In this study of 39 patients with moderate to severe plaque psoriasis with a Psoriasis Area and Severity Index (PASI) score ≥ 12, patients were randomly assigned to receive a single intravenous (IV) dose (0.01, 0.05, 0.25, 1.00, 3.00, or 5.00 mg/kg) of BI 655066 (n = 18) or placebo (n = 6), and 15 patients were randomly assigned to receive a single 0.25 mg/kg or 1.00 mg/kg dose of subcutaneous (SC) BI 655066 (n = 13) or placebo (n = 2). End points included safety; PASI at 0, 2, 4, 12, or 24 weeks; and skin biopsies for histology and next-generation RNA sequencing analysis at 0 and 8 weeks.
The mean change in PASI score was significantly higher among patients who received IV or SC BI 655066 compared with patients who received placebo, in a pooled analysis (P < .01; Figure 1).
Effect of BI 655066 on PASI Score
IV, intravenous; PASI, Psoriasis Area and Severity Index; SC, subcutaneous.
aIV and SC BI 655066 groups combined.
Reproduced with permission from J Krueger, MD, PhD.
In patients who received SC BI 655066, the mean improvement in PASI score was about 90% at week 12 and 100% at week 16; PASI-100 was maintained by 66% of patients who entered an optional long-term follow-up period for up to 66 weeks. In addition, immunohisto-chemical analysis of a skin biopsy sample harvested from a patient who received 0.25 mg/kg of SC BI 655066 demonstrated a decrease in markers associated with psoriasis at 8 weeks, including K16, K167, S100A7, Lipocalin, β-defensin, CD3, CD11c, and DC-lamp. Furthermore, next-generation sequencing analysis found that treatment with BI 655066 resulted in normalization of psoriatic lesions similar to that of nonlesional skin.
Any adverse event (AE) occurred in 20 of 31 (65%) patients who received BI 655066 and in 7 of 8 (88%) of patients who received placebo. Serious AEs, including recurrent alcoholic pancreatitis, recurrent stroke, transient ischemic attack, and myositis, occurred in 13% of patients who received BI 655066 compared with none of the patients who received placebo. Common AEs included nasopharyngitis, headache, and upper respiratory tract infection.
The results of this study show that the novel drug BI 655066 appears to be safe and well tolerated in patients with moderate to severe plaque psoriasis. In a subset of the study patients, the improvement in the secondary end point of mean change in PASI score was maintained at 1 year. This trial was not powered to evaluate efficacy end points, however. The effect of BI 655066 is being evaluated in an ongoing phase 2b study.
- © 2014 MD Conference Express®