Summary
Key issues in critical care for the acute care cardiologist were addressed by a panel of experts. Specific topics include the Berlin definition of acute respiratory distress syndrome, merging applications for extracorporeal support, intravenous fluid managementa, s well as emerging pathogens in sepsis.
- Prevention & Screening
- Bacterial Infections
- Critical Care
- Myocardial Infarction
- Exclusive Article - For home page
- Prevention & Screening
- Bacterial Infections
- Critical Care
- Cardiology
- Myocardial Infarction
- Exclusive Article - For home page
Key issues in critical care for the acute care cardiologist were addressed by a panel of experts. The Berlin definition of acute respiratory distress syndrome (ARDS) [Ranieri VM et al. JAMA. 2012] was the topic discussed by Alexandre Mebazaa, MD, University Hospital St Louis-Lariboisière, Paris, France. The new definition is simplified when compared with the former definition established in 1994 at the American-European Consensus Conference, and it characterizes ARDS by its acute onset (defined as within 7 days of a known clinical insult or new or worsening respiratory symptoms), the presence of bilateral opacities consistent with pulmonary edema on chest imaging, and respiratory failure not fully explained by cardiac failure or fluid overload. There is no use of the term acute lung injury in the Berlin definition.
The oxygenation severity scale was also simplified to include 3 categories of ARDS according to the measured PaO2: FiO2 ratio (in mm Hg) with positive end-expiratory pressure ≥ 5 cm H2O: mild (200 to 300), moderate (100 to 200), and severe (≤ 100). The severity scale correlates with mortality, noted Prof Mebazaa, with a mortality of 27% for mild ARDS, 32% for moderate, and 45% for severe.
Chiara Lazzeri, MD, Azienda Ospedaliero-Universitaria Careggi, Florence, Italy, spoke about emerging applications for extracorporeal support. The potential lifesaving indications for extracorporeal membrane oxygenation (ECMO) are expanding, as technological advances have improved ECMO circuits and made ECMO more widely available.
Considerations in the use of extracorporeal cardiopulmonary resuscitation (ECPR) are based on observational studies or those based on alternate designs, such as propensity score-matched analysis. Most of these analyses—in patients with either in-hospital or out-of-hospital cardiac arrest—demonstrated improved survival at discharge and up to 1 year, as well as improvement in the rate of survival with minimal neurologic impairment, in groups receiving either ECPR or cardiopulmonary resuscitation assisted with autopriming portable ECMO, as compared with patients receiving conventional cardiopulmonary resuscitation [Johnson NJ et al. Resuscitation. 2014; Wang CH et al. Resuscitation. 2014; Maekawa K et al. Crit Care Med. 2013; Shin TG et al. Int J Cardiol. 2013; Shin TG et al. Crit Care Med. 2011; Chen YS et al. Lancet. 2008].
The success of ECMO support depends on the selection of patients, ECMO team expertise, and ECMO postresuscitation care, said Prof Lazzeri. Factors associated with successful extracorporeal resuscitation for cardiac arrest include patient age (16 to 70 years), witnessed arrest, < 15-minute duration from collapse to emergency medical services arrival, and ventricular fibrillation or ventricular tachycardia as the initial rhythm [Johnson NJ et al. Resuscitation. 2014; Wang CH et al. Resuscitation. 2014]. Postresuscitation care that included early reperfusion when coronary arteries are occluded [Kagawa E et al. Circulation. 2012], therapeutic hypothermia, mechanical chest compression, and establishment of an intra-arterial balloon pump [Sakamoto T et al. Resuscitation. 2014; Stub et al. Resuscitation. 2014; Belohlavek J et al. J Transl Med. 2012] correlated with better outcomes.
ECMO could be the first option for resuscitation in settings where ECPR is available, Prof Lazzeri said; therefore, ECMO networks have been proposed as a means to extend mechanical circulatory support assistance, even to primary care centers. ECMO is being explored in refractory cardiogenic shock [Beurtheret S et al. Eur Heart J. 2013; Tsao NW et al. J Crit Care. 2012] and massive pulmonary embolism [Wu MY et al. Resuscitation. 2013].
Kirsten Møller, PhD, Rigshospitalet, Copenhagen, Denmark, discussed emerging pathogens in sepsis, noting the increasing prevalence of infections due to Gram-positive organisms and fungi. Emerging pathogens include methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamase-producing Gram-negative bacteria, vancomycin-resistant Enterococcus faecium, multidrug-resistant Acinetobacter baumannii, and Clostridium difficile.
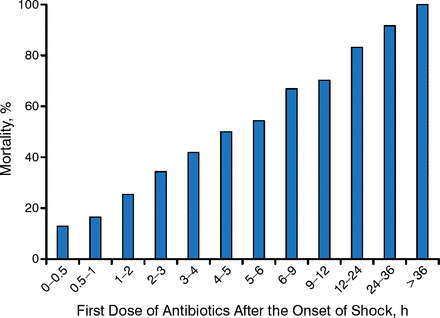
Early adequate antimicrobial coverage in septic shock is key to a successful outcome (Figure 1), she said, as institution of antimicrobials within 30 minutes is associated with a mortality rate of < 20%, while delayed antimicrobial therapy (> 36 hours) is almost always fatal [Nobre V et al. Curr Opin Crit Care. 2007; Kumar A et al. Crit Care Med. 2006]. Early goal-directed therapy - which involves adjusting cardiac preload, afterload, and contractility to balance oxygen delivery with oxygen demand—may improve overall survival. Early goal-directed therapy was associated with an improvement in 28-day mortality by 42% when compared with standard therapy [Rivers E et al. N Engl J Med. 2001].
Impact of Early Adequate Antimicrobials in Septic Shock
Reproduced from Nobre V et al. Prompt antibiotic administration and goal-directed hemodynamic support in patients with severe sepsis and septic shock. Curr Opin Crit Care. 2007;13:586–591. As adapted from Kumar A et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–1596. Used with permission from Lippincott Williams & Wilkins.
Norepinephrine is the sympathomimetic of choice in septic shock based on a review of studies comparing it with dopamine [de Backer D et al. Crit Care Med. 2012]. Blood glucose should be kept < 10 mmol/L, but intensive control to 4.5 to 6.0 mmol/L is no better and is sometimes significantly worse than conventional control on outcomes [Finfer S et al. N Engl J Med. 2009]. A transfusion hemoglobin threshold of 7 g/dL is safe in septic shock when compared with 9 g/dL, producing similar rates of mortality and ischemic events at 90 days in a recently published trial [Hoist LB et al. N Engl J Med. 2014].
Intravenous (IV) fluid management plays a fundamental role in the acute cardiac care of hospitalized patients, playing a vital role in establishing and maintaining cellular homeostasis, said Antonello Gavazzi, MD, Ospedali Riuniti di Bergamo, Bergamo, Italy. When used appropriately, IV fluid therapy can improve outcomes. Management of fluid should be accomplished per volume status at clinical stage of disease: rescue, optimization, stabilization, and de-escalation [Hoste EA et al. Br J Anaesth. 2014]. In the rescue stage, fluid bolus should be administered to correct hypotensive status. During the optimization stage, continuous infusion of fluid should be carried out to maintain homeostasis, replace losses, or prevent organ injury. In the stabilization stage, fluid should be maintained only if oral intake is inadequate. During de-escalation, IV fluid should be avoided if oral intake of fluid is possible.
Inappropriate use of IV fluid therapy may result in high levels of morbidity, prolongation of hospitalization, and excess mortality. Inappropriate use ranges from inadequate rehydration to excessive fluid infusion, leading to tissue hypoperfusion or tissue edema and severe electrolyte derangements.
A multiparametric approach should be used for the assessment of fluid status, aiming to individualize fluid management.
-
A chest radiograph can identify signs of lung congestion, such as upper-zone vessel enlargement, high vascular pedicle width, septal lines, pulmonary edema, and pleural effusions.
-
Right ventricular and left ventricular (LV) filling pressure hemodynamics can be measured with a Swan-Ganz catheter, along with cardiac output and vascular resistance.
-
Noninvasive ultrasonography can calculate the dimensions of the inferior vena cava and its variation to respiration and ventilation, as another method to estimate right atrial filling pressures and preload.
-
Natriuretic peptides maybe measured; levels of B-type natriuretic peptide (BNP) are related to LV filling pressure. BNP levels can be monitored to determine response to fluid management.
-
Carbohydrate antigen 125 is a biomarker that correlates with hemodynamic variables, fluid congestion, diastolic function, BNP, filling pressures, left atrial volume, and pleural fluids and can be modified with fluid management.
-
Bioimpedance vector analysis is a noninvasive technique to estimate body mass and water composition by bioelectrical impedance measurements, resistance, and reactance. Bioimpedance vector analysis can assess the hydration state in normal, hyperhydrated, and dehydrated patients by drawing a hydragram.
In conclusion, the knowledge, skills, tools, and data to support the critical care cardiologist continue to evolve, as does the critical care management of the intensive cardiovascular (CV) care unit patient population. The CV intensive care unit of the 21st century is not the one that your senior colleagues trained in and staffed. Programs of CV intensive care unit fellowship training are being established in many tertiary and quaternary hospitals to support the needs of acute care cardiologists and their patients.
- © 2014 MD Conference Express®