Summary
Saline-filled intragastric balloons are a possible option for patients who are not suitable candidates for bariatric surgery or do not want it. This article presents the results of A Prospective, Randomized Multicenter Study to Evaluate the Safety and Efficacy of the ReShape Duo Intragastric Balloon System in Obese Subjects [REDUCE; NCT01673698].
- Obesity
- Nutrition Clinical Trials
- Diabetes & Endocrinology Clinical Trials
- Endocrinology
- Diabetes & Metabolic Syndrome
- Obesity
- Nutrition Clinical Trials
- Diabetes & Endocrinology Clinical Trials
Saline-filled intragastric balloons are a possible option for patients who are not suitable candidates for bariatric surgery or do not want it. Jaime Ponce, MD, Hamilton Medical Center, Dalton, Georgia, USA, presented the results of A Prospective, Randomized Multicenter Study to Evaluate the Safety and Efficacy of the ReShape Duo Intragastric Balloon System in Obese Subjects [REDUCE; NCT01673698]. In the study, when the Duo system was used with diet modification and a regular exercise program, it showed overall improvements in obesity-related comorbidities and increased quality of life (QOL) in addition to measureable weight loss. Dr Ponce is the principal investigator of the trial.
In this multicenter double-blind study, patients (n = 326) with a body mass index of 30 to 40 kg/m2 with > 1 obesity-related comorbidity were randomized into 1 of 2 groups. For the first 24 weeks, the DUO group (n = 187) was treated with the Duo system (2 connected balloons delivered endoscopically), diet, and exercise. The DIET group (n = 139) was treated with diet and exercise alone. From weeks 24 to 48, the DUO group was treated with diet and exercise, while the DIET group had the option of exiting the study or having a Duo balloon inserted. The patients were counseled monthly and assessed for weight, adverse events, and QOL measures. The co-primary end points were as follows: (1) a > 7.5% excess weight loss (EWL) in the DUO group compared with the percentage EWL in the DIET group and (2) a > 35% response in the DUO group with ≥ 25% EWL.
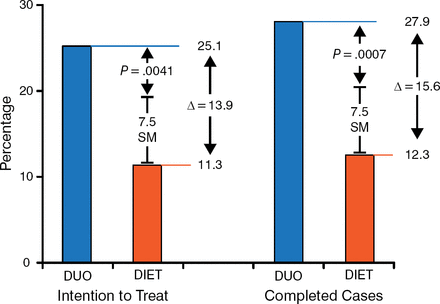
The DUO group had a significantly (P = .0041) greater percentage EWL than the DIET group and a greater proportion of ≥ 25% EWL at 24 weeks (Figure 1). Additionally, the mean percentage EWL at 48 weeks was 65% of the percentage EWL when the device was removed at 24 weeks. A total of 36% of the patients maintained a ≥ 25% EWL. Of note, about 1 in 4 patients continued to lose weight after balloon retrieval.
Primary End Point
“Percentage” indicates excess weight loss percentage. DIET, group treated with diet and exercise only; DUO, group treated with the Duo system plus diet and exercise; SM, superiority margin.
Reproduced with permission from J Ponce, MD.
Patients also showed significant improvements in laboratory measurements associated with comorbidities, including HbA1c, lipids, and blood pressure (Table 1). In addition to reporting significantly better improvement in QOL based on 3 surveys, 64% of patients said that they would repeat the Duo treatment, and 78% said that they would recommend the procedure to a friend.
Improvements in Laboratory Values Associated With Comorbidities: DUO Group (n = 187)
Most (99.6%) of the balloon insertions were successful, and all were retrieved successfully. However, 3 patients (1.1%) experienced serious adverse events following retrieval. These included pneumonia, a contained perforation of the cervical esophagus (successfully treated with antibiotics), and a proximal esophageal mucosal tear. Of the 264 patients who experienced balloon insertion, none had migration of the balloon, obstructions, or the need for surgery. The most common adverse events were mild to moderate gastrointestinal symptoms that occurred within the first 30 days and resolved quickly after the procedure. Symptoms during adjustment to the device can be treated with fluids, reassurance, and prescription medication.
Fifteen percent of the balloons had to be retrieved because of ulcers or intolerance. Given an interim analysis showing more removals in shorter patients, the researchers began to use smaller fill volumes (750 cc) for shorter patients, and this lowered intolerance by 60%. The researchers also modified the tip of the device owing to concerns that it was causing damage to the incisural wall. This change reduced the ulcer rate to 10.3%.
Dr Ponce noted some possible future uses for the Duo system. These included sequential use to increase weight loss, use in combination with medication for weight loss, and use in adolescents needing a reversible approach. Furthermore, it can assist with weight loss before surgery for patients who are not surgical candidates owing to the risks associated with a high body mass index (≥ 40).
- © 2014 MD Conference Express®