Summary
In the United States, 795,000 people experience a new or recurrent stroke each year. Approximately 610,000 of these are first attacks, and 185,000 are recurrent attacks. Mortality data from 2008 indicate that stroke accounted for 1 of every 18 deaths [Roger VL et al. Circulation 2012]. This article discusses the prevention of recurrent ischemic stroke.
- Cerebrovascular Disease
- Ischemia
- Prevention & Screening
- Cerebrovascular Disease
Secondary Prevention After Ischemic Stroke or Transient Ischemic Attack
Every year 15 million people worldwide suffer a stroke. Nearly 6 million die and another 5 million are permanently disabled. Stroke is the second leading cause of disability, after dementia. Transient ischemic attacks (TIAs) are sometimes referred to as “warning strokes” as they may be an indication that a full, far more serious stroke is about to happen [http://www.world-heart-federation.org/cardiovascular-health/stroke].
In the United States, 795,000 people experience a new or recurrent stroke each year. Approximately 610,000 of these are first attacks, and 185,000 are recurrent attacks. Mortality data from 2008 indicate that stroke accounted for 1 of every 18 deaths [Roger VL et al. Circulation 2012]. Hussien H. Rizk, MD, Cairo University Medical School, Cairo, Egypt, discussed the prevention of recurrent ischemic stroke.
The INTERSTROKE study [O'Donnell MJ et al. Lancet 2010], a standardized case-control study in 22 countries worldwide, suggested that 10 risk factors are associated with 90% of the risk of stroke. The population-attributable risk for common risk factors range from 3.8% (99% CI, 0.9 to 14.4) for alcohol intake to 34.6% (99% CI, 30.4 to 39.1) for hypertension (Table 1).
INTERSTROKE: Population-Attributable Risk for Common Risk Factors.
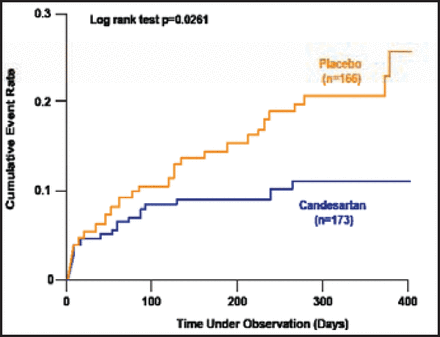
The Acute Candesartan Cilexetil Therapy in Stroke Survivors study [ACCESS; Schrader J et al. Stroke 2003] assessed the safety of modest blood pressure reduction by candesartan (an angiotensin receptor blocker) in the early treatment of stroke. Five hundred patients were recruited in a prospective, double-blind, placebo-controlled, randomized, multicenter Phase 2 safety study.
The trial was stopped prematurely when the cumulative 12-month mortality and number of vascular events differed significantly in favor of the candesartan group (OR, 0.48; 95% CI, 0.25 to 0.90). The cumulative event rates in patients receiving candesartan or placebo following an acute stroke are shown in Figure 1.
ACCESS: Cumulative Event Rate in Patients Receiving Candesartan or Placebo Following an Acute Stroke.
ACCESS=Acute Candesartan Cilexetil Therapy in Stroke Survivors.
Reprinted from Stroke, Vol 34/7, 1699–703, Schrader J et al., The ACCESS study: Evaluation of acute candesartan cilexetil therapy in stroke survivors, Copyright 2003, with permission from Lippincott Williams & Wilkins.
The Perindopril Protection Against Recurrent Stroke Study [PROGRESS] determined the effects of a blood pressure–lowering regimen in hypertensive and nonhypertensive patients with a history of stroke or TIA [PROGRESS Collaborative Group. Lancet 2001]. The primary outcome was total stroke (fatal or nonfatal).
A total of 6105 individuals from 172 centers in Asia, Australasia, and Europe were randomly assigned active treatment (n=3051) or placebo (n=3054). Active treatment was a flexible regimen based on the angiotensin-converting enzyme inhibitor perindopril (4 mg QD), with the addition of the diuretic indapamide at the discretion of treating physicians.
Over 4 years of follow-up, active treatment reduced blood pressure by 9/4 mm Hg on average. Combination therapy with perindopril plus indapamide reduced blood pressure by 12/5 mm Hg and stroke risk by 43% (95% CI, 30 to 54). Single-drug therapy reduced blood pressure by 5/3 mm Hg and produced no discernible reduction in the risk of stroke.
Davis and Donnan [N Engl J Med 2012] noted that investigations (including brain imaging and arterial cardiac assessment) are warranted promptly after a TIA or stroke to determine cause and guide interventions to reduce subsequent risk. Physicians should routinely pay attention to lifestyle factors and prescribe blood pressure-lowering, statin, and antiplatelet drugs as indicated. Effective secondary-prevention strategies for selected patients include carotid revascularization for high-grade carotid stenosis and anticoagulation therapy for atrial fibrillation.
- © 2012 MD Conference Express®