Summary
Today, the study of diabetes is at the crossroads of insulin signaling and insulin resistance as the scientific community seeks to better understand insulin action, sites of regulation, and unoccupied receptor signaling. This article discusses some of the most recent research regarding insulin action and insulin resistance.
- diabetes mellitus
- insulin
It has been 93 years since the discovery of insulin. Before its discovery in 1921, patients with type 1 diabetes usually died within months after onset. As of 2014, more than 5000 individuals have survived 50 years or more with insulin-dependent diabetes, some having lived with diabetes for ≥ 85 years.
Today, the study of diabetes is at the crossroads of insulin signaling and insulin resistance as the scientific community seeks to better understand insulin action, sites of regulation, and unoccupied receptor signaling. During the Presidential Plenary of the 2014 joint meeting of the International Congress of Endocrinology (ICE) and the Endocrine Society (ENDO), C. Ronald Kahn, MD, Joslin Diabetes Center and Harvard Medical School, Boston, Massachusetts, USA, discussed some of the most recent research regarding insulin action and insulin resistance.
The insulin/insulin-like growth factor (IGF-1) signaling network is of paramount importance in metabolic diseases such as diabetes, obesity, and metabolic syndrome. Dr. Kahn reviewed the basic concept of a ‘critical node’ as it applies to this important pathway. A critical node within any complex, physiologically important cell-signaling network is an essential point of regulation, signal divergence, and crosstalk with other signaling cascades [Taniguchi CM et al. Nat Rev Mol Cell Biol 2006]. At least three critical nodes—insulin receptor (IR)/insulin receptor substrate (IRS), phosphatidylinositol-3 kinase (PI3K), and AKT/protein kinase-B (PKB)—have already been identified as playing an important role in diabetes and obesity.
More recently, the classical model of the ligand-activated tyrosine kinase-dependent pathway for insulin receptor signaling has been replaced by a model in which the insulin or IGF-1 receptor signal can be modulated by other membrane proteins or co-receptors and in which the unoccupied receptor may also create independent signals. In this newer model, proteins in membrane or serum are bound or activated by IR and IGF-1, which both act as anti-apoptotic hormones.
Data obtained from a study of double-knockout (DKO) mice cells that lacked both insulin and IGF-1 receptors and subsequently became resistant to apoptosis induced through either the intrinsic or extrinsic pathways suggest that the presence of unoccupied IRs and IGF-1 somehow are permissive for normal cellular apoptosis and that when these receptors are missing, apoptosis is markedly reduced [Boucher J et al. Sci Signal 2010]. The resistance was confirmed by measuring several different parameters, including DNA fragmentation, caspase 3 cleavage, and annexin binding. The resistance was associated with a decrease in the proapoptotic protein Bax and an increase in four antiapoptotic proteins: Bcl-2, Bcl-xL, XIAP, and Flip. Sensitivity to apoptosis was regained when the deleted IR and IGF-1 receptors were restored.
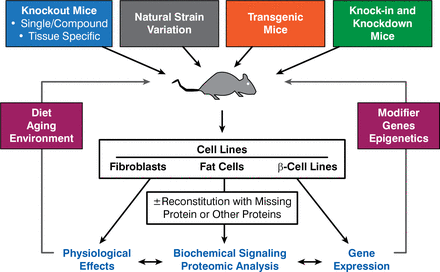
Dr. Kahn discussed his work using genetically engineered mice and the use of RNA interference in both cells and in intact animals to better define the role of IR and various downstream molecules in insulin action and insulin resistance (Figure 1). For example, the creation of p85αMut knock-in mice has allowed researchers to study a syndrome similar to the SHORT syndrome. SHORT—a rare condition characterized by multiple abnormalities that affect several parts of the body—is a mnemonic for short stature, hyperextensibility, ocular depression, Rieger anomaly, and teething delay. Other characteristics include insulin resistance and decreased visceral/subcutaneous fat.
Strategies for Mouse and Cellular Models to Study Insulin Signaling
Reproduced with permission from CR Kahn, MD.
Until now, there have been no good human cell models with which to study insulin resistance because of a general lack of human cell lines that respond to insulin. However, human insulin resistance can now be studied using a model based on induced pluripotent stem cells (iPSCs), which can be generated directly from adult cells. iPSCs hold great promise in the field of regenerative medicine because they give rise to every other cell in the body [Takahashi K et al. Cell 2006]. Dr. Kahn described the creation of the first iPSCs that offer a human model of insulin resistance [Iovino S et al. Diabetes 2014]. In this case, the original cells were fibroblasts—connective tissue cells derived from skin samples. Some of these cells were then ‘reprogrammed’ into iPSCs that would likely respond to insulin. Both sets of fibroblasts and the corresponding iPSCs were then analyzed for insulin signaling and gene expression. iPSCs from patients with IR mutations showed altered insulin signaling and altered gene expression. Insulin resistance also reduced iPSC proliferation and the expression of early genes, perhaps representing an unrecognized mechanism that is important to the development of diabetes.
In closing, Dr. Kahn reiterated that the study of diabetes is at a crossroads. New tools and new concepts are needed to further understand insulin signaling and insulin resistance.
- © 2014 MD Conference Express®