Summary
Although surgical aortic valve replacement is recommended by clinical practice guidelines in patients with severe aortic stenosis, many patients with calcific aortic stenosis are not optimal candidates for surgery due to their medical comorbidities. Transcatheter aortic valve replacement (TAVR) offers an alternative approach for this patient population that is less invasive. This article discusses indications and outcomes TAVR in patients with severe aortic stenosis, as well as strategies for appropriate patient selection for TAVR.
- Interventional Techniques & Devices
- Valvular Disease
- Interventional Techniques & Devices
- Valvular Disease
- Cardiology
Although surgical aortic valve replacement (AVR) is recommended by clinical practice guidelines in patients with severe aortic stenosis, many patients with calcific aortic stenosis are not optimal candidates for surgery due to their medical comorbidities. Transcatheter aortic valve replacement (TAVR) offers an alternative approach for this patient population that is less invasive. Martine Gilard, MD, Brest Centre Hospitalier Regional Universitaire, Brest, France, presented indications and outcomes TAVR in patients with severe aortic stenosis.
An important aspect of TAVR is appropriate patient selection. A multidisciplinary heart team is important to fully evaluate the patient, develop an individual risk profile and to determine anatomic suitability of TAVR. Prof. Gilard stated that the multidisciplinary heart team should consist of surgeons, cardiologists, anesthesiologists, imaging specialists, and other specialities, such as geriatricians [Vahanian A et al. Eur Heart J 2012].
Current indications for TAVR include severe, symptomatic aortic stenosis in patients with a life expectancy of ≥1 year who are not optimal surgical candidates. Prof. Gilard stated that comorbidities such as chronic obstructive pulmonary disease (COPD), concomitant coronary artery disease, and obesity can cause symptoms similar to those seen in severe aortic stenosis and must be ruled out prior to pursuing TAVR.
In determining feasibility of TAVR in a patient, the diameter, tortuosity, and calcifications for the transvascular approach should be evaluated via computed tomography (CT), angiography or magnetic resonance imaging (MRI). The diameter of the aortic annulus must to be determined through imaging studies and coronary angiography should be undertaken to identify potential revascularization.
Prof. Gilard highlighted that some contraindications for TAVR include absence of a heart team or cardiac surgeon, life expectancy of <1 year, unlikelihood of improvement in quality of life, and anatomical limitations such as inadequate annulus size, presence of a thrombus in the left ventricle, endocarditis, and plaques with mobile thrombi in the ascending aorta or arch [Vahanian A et al. Eur Heart J 2012]. Other comorbidities such as bicuspid or noncalcified valves, untreated coronary artery disease that requires revascularization, hemodynamic instability, and left ventricular ejection fraction of <20% are relative contraindications to TAVR. The the potential risks and benefits of TAVR in patients with these comorbidities should be assessed by the heart team.
The PARTNER A study was a noninferiority trial that evaluated TAVR and surgical AVR in high- risk surgical patients. In this trial, the mortality of patients who received TAVR was similar to patients who received surgical AVR at 12 months (HR 0.93, 95% CI, 0.71 to 1.22, p=0.62) [Smith CR et al. N Engl J Med 2010]. In the PARTNER B trial which randomized patients with severe aortic stenosis who were not candidates for surgical AVR to either medical therapy or TAVR, 1-year mortality was significantly lower in patients who underwent TAVR (67.6% vs. 43.3) [Leon MB et al. N Engl J Med 2011].
According to data from several European registries, major complications experienced by patients who underwent TAVR included major vascular complications (3.3% to 6.3%), requirement of a new pacemaker (13% to 39.3%), bleeding and tamponade (up to 17.7%), and stroke (1.2% to 5%). In the German Aortic Valve Registry (GAVR), data from 13,860 patients were analyzed and stratified by type of AVR (surgical vs transcatheter), performance of AVR plus coronary artery bypass grafting (CABG) versus AVR alone, and type of percutaneous approach to TAVR (femoral vs transapical). Cerebrovascular events occurred in 2.2% of patients who received AVR only, 3.6% of patients who received surgical AVR plus CABG, 3.7% of patients who received femoral TAVR, and 3.5% of patients who received transapical TAVR. A new pacemaker was required in 4.6% and 3.9% of patients who received surgical AVR only and surgical AVR plus CABG, respectively, as compared with 23.7% and 9.9% of patients who underwent femoral or transapical TAVR, respectively. In-hospital mortality was 2.1%, 4.5%, 5.1%, and 7.7% in patients who underwent surgical AVR only, surgical AVR plus CABG, femoral TAVR, and transapical TAVR, respectively.
In a follow-up study of 88 patients who had received TAVR, the cumulative survival steadily decreased over the study period of 5 years, with a 1-year survival rate of 83% and a 5-year survival rate of 35% [Toggweiler S et al. J Am Coll Cardiol 2013]. Patients with COPD and moderate or greater paravalvular regurgitation after TAVR had a lower rate of survival.
Prof. Gilard concluded that TAVR should be used as the standard of care in patients who are unable to undergo surgical AVR or in high-risk patients in whom the heart team feel TAVR would result in better outcomes than surgical AVR. Future directions include the development of a more accurate measure of risk in potential TAVR patients and methods to decrease paravalvular leaks and stroke following the TAVR.
Although TAVR appears to offer a promising alternative to surgical AVR for patients who are poor surgical candidates, Bernard Chevalier, MD, Institut Cardiovasculaire Paris Sud, Massy, France, presented strategies for appropriate patient selection for TAVR, as he pointed out that there are clearly differences in outcomes based on patient selection.
One characteristic that may be important in patient selection for TAVR is frailty. Indications of frailty may include number and type of comorbidities, combined into a score where comorbidities such as metastatic cancer is weighted 5; congestive heart failure 2; weight loss 2; alcohol abuse 1; cardiac arrhythmias 1; and liver disease 1. Another score uses the combined score from three tests: grip strength, walking speed, and chair rise time. The Katz daily life score, which measures disability, assesses the ability of a patient to take a bath, get dressed, get washed, go from their bed to a chair, and to prepare a meal, where 0 is given for easy to do alone and 4 is given for impossible to do alone.
Prof. Chevalier suggested that another characteristic for patient selection includes analysis of the ilio-femoral vessels, which are required for vascular access during TAVR. Important features to be aware of are vessel sinuosities, angles, vessel diameter, and the presence of plaque and calcification. Location of vessel access may also be important to consider. In the PARTNER Cohort A study, mortality rates 30 days post procedure were 3.7% versus 8.2% in patients who underwent transfemoral TAVR versus surgical AVR (p=0.046), while the mortality rate in patients who received transapical arm TAVR or AVR was 8.7% and 7.6%, respectively (p=0.79) [Smith CR et al. N Engl J Med 2011]. Recent case series have described an alternative approach in which the valve is inserted via a transaortic approach. The potential advantages of a transaortic approach for TAVR include frequent use of aortic annulation and ministernotomy, no left ventricular access, less chest wall complications, and the ability to convert to a full sternotomy if required. Across three studies, the observed mortality rate of TAVR via the transaortic access ranged from 6.9% to 10.9% [Hayashida K et al. Eur J Cardiothorac Surg 2013].
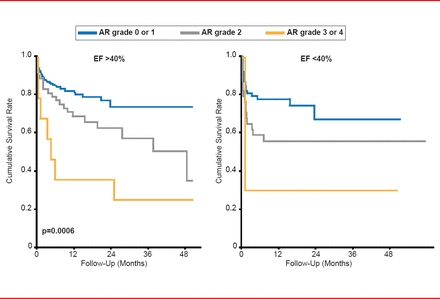
Valve selection is also an important feature to consider prior to TAVR. In multiple international studies, aortic regurgitation (AR) was a prominent occurrence following TAVR, ranging from 13.1% to 21% of patients. Importantly, the survival rate following TAVR decreases as AR grade worsens, regardless of ejection fraction (Figure 1). Prof. Chevalier suggested that the shape of the aortic valve annulus and its diameter may be important indicators of AR. A recent study demonstrated that CT-guided versus transesophageal echocardiography (TEE)-guided valve sizing was likely more accurate, resulting in less AR and annulus rupture [Hayashida K et al. EuroIntervention 2012].
Effect of Aortic Regurgitation on Survival After TAVR
AR=aortic regurgitation; EF=ejection fraction.
Reproduced with permission from B Chevalier, MD.
In conclusion, Prof. Chevalier stated that global patient evaluation is critical and a collaborative decision by the heart team is necessary prior to TAVR. In addition, anatomical screening is an important aspect of patient screening. Prof. Chevalier emphasized that the transaortic approach is promising and that valve selection is an important characteristic that can affect patient outcomes.
- © 2013 MD Conference Express®