Summary
A balance between a normal rate of endothelial cell apoptosis and regeneration is necessary to maintain the endothelial layer and protect the cardiovascular system against atherosclerosis. Similarly, an imbalance between life and death of the myocyte following an acute myocardial infarction might explain post-AMI remodeling and consequent progression of heart failure.
- myocardial infarction
- coronary artery disease
A balance between a normal rate of endothelial cell apoptosis and regeneration is necessary to maintain the endothelial layer and protect the cardiovascular (CV) system against atherosclerosis. When a mismatch occurs and apoptosis exceeds regeneration, the imbalance causes discontinuity of the endothelial layer, favoring the initiation and progression of a biochemical sequence that leads to atherosclerosis, plaque rupture, and eventually acute coronary syndromes (ACSs). Similarly, an imbalance between life and death of the myocyte following an acute myocardial infarction (AMI) might explain post-AMI remodeling and consequent progression of heart failure (HF). Roberto Ferrari, MD, University of Ferrara, Lumezzane, Italy, stated that “the life and death of the endothelium and of the myocyte” are all part of the CV continuum.
He described the cardiovascular disease (CVD) continuum as a sequence of related pathological events that start with risk factors, such as hypertension, dyslipidemia, insulin resistance, and smoking. These events may then lead to endothelial dysfunction, atherosclerosis, and coronary artery disease (CAD), which in turn might result in myocardial thrombosis and infarction, arrhythmia and loss of muscle, cardiac remodeling, ventricular dilation, congestive HF (CHF), and end-stage heart disease.
The endothelium undergoes the life (reproduction)-and-death (apoptosis) cycle every 3 months, and its function is altered when apoptosis exceeds reproduction from the bone marrow. Endothelial function can be manipulated by interfering with the life-and-death cycle. Apoptosis can be measured by incubating human umbilical vein endothelium cells (HUVECs) with serum from normal individuals or patients with CAD of different severities. Using this method, the rate of endothelial apoptosis in CAD patients was reported to be significantly higher for patients with stable angina and significantly more so for ACS patients compared with healthy volunteers [Ferrari R et al. Circulation 2003]. This was associated with a parallel downregulation of eNOS protein expression and activity, an established index of endothelial function.
Successful control of endothelial function and apoptosis can be achieved with ACE inhibitors and statins. PERTINENT, the substudy of [Cardiovascular Drugs & Therapy 2003] the EUropean trial on Reduction Of cardiac events with Perindopril in stable coronary Artery disease (EUROPA) showed that perindopril normalized the angiotensin II/bradykinin balance, reduced inflammation, and prevented endothelial apoptosis. One year of treatment with perindopril upregulated eNOS protein expression and activity (19% and 27% vs placebo; p<0.05) and reduced the rate of apoptosis by 31% (p<0.05). Similarly, von Willebrand factor, another index of endothelial function, was significantly reduced (p<0.001). Increased endothelial apoptosis in CAD patients was accompanied by excess angiotensin II and tumor necrosis factor-alpha (TNFα) and a reduction in bradykinin, which were all reversed by perindopril treatment [Ceconi C et al. Cardiovasc Res 2006; Ferrari R & Fox K. Drugs 2009].
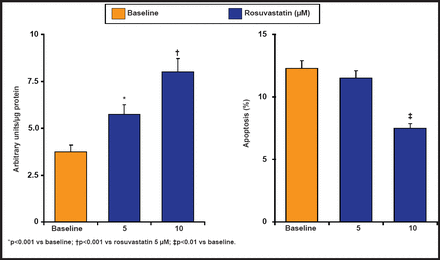
Similar effects on eNOS expression and apoptosis have been shown in HUVECs that have been incubated with rosuvastatin (Figure 1). In addition, rosuvastatin significantly reduced apoptosis in patients with ACS after 48 hours. Rosuvastatin exerts its antiapoptotic effect by inhibiting procaspase 9, BAD-Bcl-2 (Bcl-2-associated death promoter protein), and the activation of Akt signaling pathways. In a carotid artery injured mouse model, 10 days of pretreatment with rosuvastatin increased circulating bone marrow-derived progenitor cells, enhancing vascular reendothelialization and reducing neointimal formation [Werner N et al. Arterio Thromb Vasc Biol 2002].
eNOS Expression in CAD Patients.
Reproduced with permission from R. Ferrari, MD.
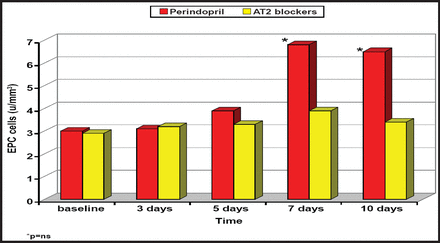
Mature endothelial cells possess limited regenerative capacities; however, there is recent evidence that reduced availability of endothelial progenitor cells (EPCs) or impairment of their function may be associated with more severe CVD and comorbid risk factors, suggesting that EPCs may offer a new avenue of treatment. These cells are able to locate the site of heart injury/damage and may play a part in angiogenesis. Unlike the angiotensin II receptor antagonists, perindopril significantly increases EPCs in MI patients after 7 days of treatment (Figure 2).
MI Patients.
Reproduced with permission from R. Ferrari, MD.
This phenomenon occurs mainly as a consequence of a large infarction and consists of two main phases: 1 – early remodeling, which occurs at the site of myocardial damage, resulting in the thinning of the ventricular wall, scar formation, and subsequent enlargement and reshaping of the ventricular chamber; 2 – late phase remodeling, which might occur months or even years after the initiating insult in the still viable myocytes (ie, in the region of the ventricle that is not affected by the infarction). While the first phase of remodeling, which includes a repair process that leads to the formation of a scar, may be considered a favorable response to vascular injury, the second phase, involving viable myocytes, is deleterious and responsible for the progression of the syndrome. Remodeling myocytes show a typical switch forward the embryonic phenotype (ie, they reexpress atrial natriuretic peptides in the ventricles, embryonic myofilaments, and Ca++-related proteins) and classical features of apoptosis and/or hypertrophy. Interestingly, these two processes, although activated and regulated by similar intracellular cascades, represent opposite signals for the myocytes: a signal of death—apoptosis—and a signal of life—hypertrophy. This is not at all surprising, as the so-called “cell life and death cycle” is an intrinsic component of nature itself. Almost every cell of the organism undergoes “the cell cycle” (eg, a red blood cell lives for 120 days; a neutrophil for 7 hours). The adult myocyte, however, is a terminal cell; usually, it is not able to reproduce, and death is not genetically programmed (apoptosis) but occurs by necrosis as a consequence of a nonexpected event (eg, the occlusion of a coronary artery by a thrombus). The embryonic myocyte, contrary to the adult one, undergoes the full cell cycle: it dies by apoptosis and has the ability to reproduce. The reinstatement of apoptosis and development of hypertrophy could be part of the switch forward to the embryonic phenotype with reinstatement of the early embryonic genetic program. Thus, hypertrophy and apoptosis can be considered as “sons” of the same “mother:” the local, tissue neuroendocrine-neurohumoral response to a mechanical stretch of the myocytes. The stretch is consequent to the geometric changes that are imposed on the viable myocytes by the necrotic ones.
Recognized stimuli for the switch are angiotensin II, norepinephrine, and aldosterone, although many other inducers are likely to play a role. This explains the antiremodeling effect of ACE inhibitors, β-blockers, and antialdosterone substances. As expected, the life-and-death cycle is very closely regulated by several autocrine systems, one of which is linked to the interleukin 6 family via the regulatory protein GP-130. Activation of GP-130 slows down the death signals, thus favoring hypertrophy and reducing fibrosis. Although hypertrophic myocytes can not be considered normal, it has been suggested that when they are matching the myocyte loss, CHF is in a steady state. However, when apoptosis prevails over hypertrophy, the disease progresses forward to terminal stages. This “molecular-genetic” view of the remodeling processes is interesting not only from the physiopathological point of view but also from a therapeutic one, suggesting that antiapoptotic and prolife agents could be considered in the near future as novel treatments for CHF.
Prof. Ferrari said that he believes that excess endothelial apoptosis is a primum movens of plaque instability and that improvement of endothelial function may be obtained by interfering with the life-and-death cycle. Different currently available drug regimens are able to influence bone marrow production and the release of EPCs and may offer possible new therapeutic approaches.
- © 2011 MD Conference Express