Summary
The recently published 2015 European Society of Cardiology guidelines have been updated based on the wealth of new evidence from clinical trials in the different specialties. These timely and comprehensive guidelines offer guidance for clinicians on the latest management strategies for ventricular arrhythmias and sudden cardiac death, pulmonary hypertension, infective endocarditis, and acute coronary syndromes without ST-segment elevation.
- acute coronary syndromes
- European Society of Cardiology
- guidelines
- infective endocarditis
- NSTE
- pulmonary hypertension
- recommendations
- sudden cardiac death
- ventricular arrhythmia
The European Society of Cardiology (ESC) launched 5 new guidelines developed by expert task forces and peer reviewers, covering the following topics: pericardial diseases, ventricular arrhythmias (VAs) and sudden cardiac death (SCD), pulmonary hypertension (PH), infective endocarditis (IE), and non–ST-segment elevation acute coronary syndromes (NSTE-ACS). These guidelines summarize all of the available data on the topics, providing a valuable resource for practicing clinicians.
Guidelines on Pericardial Diseases
Development of the 2015 Pericardial Diseases Guidelines was led by Yehuda Adler, MD, Chaim Sheba Medical Center, Ramat-Gan, Israel, and Philippe Charron, MD, Hopital Ambroise Pare, Boulogne-Billancourt, France. The focus of the new guidelines is on diagnostic and treatment strategies [Adler Y et al. Eur Heart J. 2015].
The guidelines recommend a simple etiologic classification of pericardial diseases as infectious or noninfectious. The major causes of pericardial disease are viruses (in the developed world), bacterial pericarditis (especially tuberculosis), neoplastic pericarditis, and pericarditis associated with systemic disease (generally autoimmune disease). The classical pericardial syndromes include pericarditis, pericardial effusion, cardiac tamponade, and constrictive pericarditis. Pericardial effusion and cardiac tamponade may occur without pericarditis.
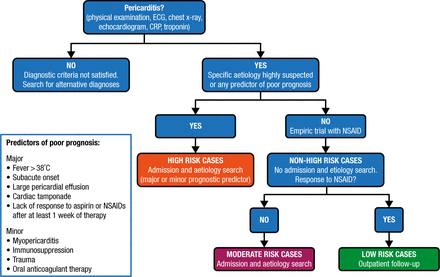
The definition of acute pericarditis is the presence of at least 2 of the following criteria: pericarditic chest pain, pericardial rubs, new widespread ST-elevation or PR depression on an electrocardiogram (ECG), and pericardial effusion. Incessant pericarditis is defined as pericarditis lasting for > 4 to 6 weeks but < 3 months without remission. Recurrent pericarditis is an episode occurring after a documented first episode of acute pericarditis and a symptom-free interval of ≥ 4 to 6 weeks. Pericarditis lasting for > 3 months is considered chronic (Figure 1).
Proposed Triage of Pericarditis
CRP, C-reactive protein; ECG, electrocardiogram.
Reprinted from Adler Y et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015; Epub ahead of print. By permission of European Society of Cardiology.
Investigations recommended for the diagnosis of pericarditis are shown in Table 1.
First- and Second-Level Investigations for Pericarditis
Pericardial effusion is classified according to onset (acute, subacute, or chronic), size (mild < 10 mm, moderate 10-20 mm, or large > 20 mm), distribution (circumferential or loculated), and composition (transudate or exudate). The etiology may be idiopathic, malignant, infectious, iatrogenic, or connective tissue disease. Evaluation of suspected pericardial effusion should include transthoracic echocardiography, chest x-ray, assessment of markers of inflammation, and computed tomography or cardiac magnetic resonance imaging in patients with loculated pericardial effusion, pericardial thickening and masses, or associated chest abnormalities.
Common causes of cardiac tamponade include pericarditis, tuberculosis, iatrogenic, trauma, and neoplasms. Echocardiography is recommended as the first imaging modality to evaluate the size, location, and degree of hemodynamic impact of pericardial effusion. Urgent pericardiocentesis or surgery is recommended to treat cardiac tamponade.
Constrictive pericarditis can occur after almost any pericardial disease but is rare following recurrent pericarditis. The most common causes are idiopathic or viral, cardiac surgery, radiation therapy, connective tissue disorder, or postinfection. Transthoracic echocardiography (TTE) and chest x-ray are recommended for all patients with suspected constrictive pericarditis.
Table 2 summarizes the recommendations for the management of acute and recurrent pericarditis, pericardial effusion (including tamponade), and constrictive pericarditis.
Recommendations for the Management of Pericarditis, Pericardial Effusion, and Constrictive Pericarditis
VAs and SCD
Carina Blomstrom-Lundqvist, Uppsala University, Uppsala, Sweden, and Silvia G. Priori, MD, PhD, University of Pavia, Pavia, Italy, led the development of the ESC Guidelines on Ventricular Arrhythmias and Sudden Cardiac Death [Priori SG et al. Eur Heart J. 2015]. This update of the 2006 guidelines focuses on preventing SCD among patients with VA.
Cardiac diseases associated with SCD are predominantly channelopathies, cardiomyopathies, myocarditis, and substance abuse in the young, and chronic degenerative diseases in older persons. For the first time, DNA analysis has been recommended as part of the standard autopsy to identify the presence of channelopathies.
Tests recommended for screening patients with suspected or known VA are shown in Table 3.
Screening of Patients With Suspected or Known Ventricular Arrhythmias
Recommended device therapies for patients with VA include implantable cardioverter defibrillators (ICDs), subcutaneous defibrillators, and wearable cardioverter defibrillators. ICD therapy is recommended for secondary prevention of SCD and ventricular tachyarrhythmia (VT) and for primary prevention of SCD in patients with severe left ventricular dysfunction. A subcutaneous defibrillator may now be considered as an alternative treatment for VA for young patients or those with limited venous access or infection. It is not suitable for patients who need bradycardia pacing, cardiac resynchronization therapy (CRT), or for patients with tachyarrhythmias that can be terminated by antitachycardia pacing. A wearable cardioverter defibrillator may now be considered for patients at transient risk for SCD but who cannot use an ICD.
Catheter ablation is recommended for patients with scar-related heart disease with incessant VT or electrical storm and for patients with ischemic heart disease and recurrent ICD shocks due to sustained VT. The new guidelines have added a recommendation to consider ablation after a first episode of sustained VT in patients with ischemic heart disease and an ICD.
CRT recommendations for primary prevention of SCD among selected patients in sinus rhythm with NYHA functional class III or ambulatory class IV and those with NYHA class II heart failure are shown in Table 4.
Cardiac Resynchronization Therapy for Primary Prevention of Sudden Cardiac Death in Patients With Sinus Rhythm
The guideline provides tables of recommendations for the treatment of cardiomyopathies.
The new guidelines include updated diagnostic criteria and management recommendations for inherited primary arrhythmia syndromes. For long QT syndrome, the evidence supports the use of ICDs in survivors of cardiac arrest. Prophylactic ICD therapy may be considered in high-risk patients. Survivors of cardiac arrest with short QT syndrome should receive ICD therapy for secondary prevention. ICD therapy is recommended for survivors of cardiac arrest with Brugada syndrome or catecholaminergic polymorphic ventricular tachycardia. Medical therapy is also recommended for patients with these syndromes.
Guidelines on PH
The ESC/European Respiratory Society (ERS) Guidelines on Pulmonary Hypertension, chaired by Nazzareno Galiè, MD, University of Bologna, Bologna, Italy, and Marc Humbert, MD, PhD, Universite Paris-Sud, Le Kremlin-Bicetre, France, address the main clinical characteristics, issues in diagnosis, and latest treatment strategies [Balie N et al. Eur Heart J. 2015]. PH can involve multiple clinical conditions and can complicate most cardiovascular and respiratory diseases. PH is defined as an increase in mean pulmonary arterial pressure ≥ 25 mm Hg at rest, as assessed by right heart catheterization (RHC). The definitions of PH and precapillary PH have not changed in the new guidelines but the definition of postcapillary PH has been revised (Table 5).
Hemodynamic Definitions of Pulmonary Hypertension
The new clinical classification for PH includes new conditions, recently identified gene mutations, and several other changes from the previous guidelines. The main classifications are: 1) pulmonary arterial hypertension (PAH), 2) PH due to left heart disease, 3) PH due to lung diseases or hypoxia, 4) chronic thromboembolic pulmonary hypertension (CTEPH) and other pulmonary artery obstructions, and 5) PH with unclear or multifactorial mechanisms.
The diagnosis of PH is based on symptoms, physical examination, review of investigations that meet hemodynamic criteria, etiology, and functional and hemodynamic severity. The main cause of PH should be identified according to the clinical classification.
RHC is recommended to confirm the diagnosis of PAH and to support treatment decisions. It is also recommended for patients with PH due to left heart diseases, lung diseases, or CTEPH. Vasoreactivity testing during RHC is recommended for patients with idiopathic, heritable, and drug- or toxin-induced PAH to identify patients who can be treated with high-dose calcium channel blockers. PAH severity should be evaluated by clinical assessment, exercise tests, biochemical markers, and echocardiographic and hemodynamic evaluation, with follow-up assessments every 3 to 6 months for stable patients. Patients with PAH should avoid pregnancy.
Initial treatment with drug monotherapy or combination drug therapy for treatment-naïve and low- or intermediate-risk patients with PAH is recommended. Initial combination therapy including an intravenous prostacyclin analogue is recommended for high-risk patients. Approved therapies for PAH are not recommended for patients with PH due to left heart disease or lung diseases. For patients with CTEPH, surgical pulmonary endarterectomy in deep hypothermia circulatory arrest is recommended.
Guidelines on IE
The 2015 ESC Guidelines on Infective Endocarditis, presented by Gilbert Habib, MD, La Timone Hospital, Marseille, France, and Patrizio Lancellotti, MD, University of Liege Hospital, Liege, Belgium, focus on increased prevention, management by a multidisciplinary team, multimodality imaging for diagnosis, new diagnostic criteria, and early surgery [Habib G et al. Eur Heart J. 2015].
The new guidelines place an emphasis on prevention of IE rather than antibiotic prophylaxis. The use of prophylaxis is still recommended for patients with predisposing cardiac conditions undergoing procedures associated with a risk of IE. Patients at highest risk of IE are those with a prosthetic valve, previous IE, or congenital heart disease. Antibiotic prophylaxis should only be considered for dental procedures requiring manipulation of the gingival or periapical region of the teeth or perforation of the oral mucosa. Good oral hygiene and regular dental review are more important to reduce the risk of IE. Susceptible patients undergoing high-risk dental procedures should receive amoxicillin or ampicillin, or clindamycin if they are allergic to penicillin. Antibiotic prophylaxis is not recommended for respiratory tract, gastrointestinal, urogenital, or skin and soft tissue procedures.
The management of IE by a multidisciplinary medical-surgical team using a standardized protocol to treat IE has been associated with a significant decrease in mortality [Botelho-Nevers E et al. Arch Intern Med. 2009]. The guidelines recommend that patients with complicated IE should be evaluated and managed at an early stage in a reference center with surgical facilities and an endocarditis team including an infectious disease specialist, microbiologist, cardiologist, imaging specialists, cardiac surgeon, and if needed, a coronary heart disease specialist.
TTE is recommended as the first-line imaging modality for diagnosis of suspected IE, followed by transesophageal echocardiography (TEE). TEE should be the first imaging modality for patients with a prosthetic valve or intracardiac device. The guideline provides a diagnostic algorithm to diagnose IE, along with modified diagnostic criteria.
The updated guidelines recommend early surgery for the treatment of IE. Heart failure is the most frequent complication of IE and is the most common indication for surgery. The second and third most common indications for surgery are uncontrolled infection and prevention of embolism, respectively.
Guidelines on ACS Without ST-Segment Elevation
Marco Roffi, MD, University Hospital, Geneva, Switzerland, discussed the new 2015 ESC guidelines for the management of acute coronary syndromes (ACS) in patients without persistent ST-segment elevation [Roffi M et al. Eur Heart J. 2015]. He focused on updates regarding diagnosis, cardiac rhythm monitoring, risk stratification, and therapeutic strategy.
Patients presenting with suspected NSTEMI should be evaluated by measuring cardiac troponins with sensitive or high-sensitivity assays; results should be obtained within 60 minutes of presentation. In addition to the rapid rule-out protocol at 0 and 3 hours, already recommended in the 2011 ESC guidelines, a rapid rule-out and rule-in protocol at 0 and 1 hour is recommended if a high-sensitivity cardiac troponin test with a validated 0 hour/1 hour algorithm is available. Additional testing after 3 to 6 hours is indicated if the first 2 troponin measurements are not conclusive and the clinical condition is still suggestive of ACS.
Continuous rhythm monitoring is recommended until the diagnosis of NSTEMI is established or ruled out. Patients with NSTEMI should be admitted to a monitored unit. Rhythm monitoring up to 24 hours or percutaneous coronary intervention (PCI), whichever comes first, should be considered for patients with NSTEMI at low risk for cardiac arrhythmias. Rhythm monitoring for ≥ 24 hours should be considered for patients with NSTEMI at intermediate to high risk for cardiac arrhythmias. In the absence of signs or symptoms of ongoing ischemia, rhythm monitoring may be necessary only in selected patients with unstable angina (ie, high-sensitive cardiac troponin negative individuals).
The treatment strategy and timing for patients with NSTE-ACS should be selected according to initial risk stratification. The novel risk stratification criteria are shown in Table 6.
Criteria for Risk Stratification in Patients With NSTE-ACS
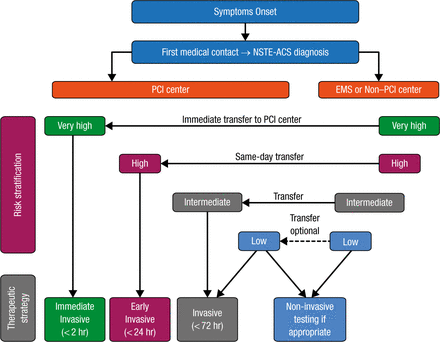
Most patients with NSTE-ACS are managed with invasive coronary angiography, followed by coronary revascularization if indicated. An algorithm for the selection of the treatment strategy and timing according to initial risk stratification is shown in Figure 2.
Selection of NSTE-ACS Treatment Strategy and Timing According to Initial Risk Stratification
EMS, emergency medical services; PCI, percutaneous coronary intervention; NSTE-ACS, non-ST segment elevation acute coronary syndrome.
Reprinted from Roffi M et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015; Epub ahead of print. By permission of European Society of Cardiology.
A recent randomized study including > 8000 ACS patients demonstrated that PCI performed using a radial access results in lower rates of non–coronary artery bypass graft major bleeding; the composite of death, myocardial infarction (MI), or stroke; and death [Valgimigli M et al. Lancet. 2015]. A meta-analysis of the randomized trials including the ones in the meta-analysis by Valgimigli and colleagues confirmed the reduction in bleeding events, vascular complications, and mortality. Based on the evidence, the 2015 guidelines recommend that centers treating patients with ACS implement a transition from femoral to radial access for coronary angiography and PCI. For patients undergoing PCI, placement of new-generation drug-eluting stents (DESs) is recommended. Even if a short dual antiplatelet therapy duration (30 days) is planned because of increased bleeding risk, a new-generation DES may be considered over a bare-metal stent.
The new ESC guidelines summarize the evidence on diagnostic and management strategies, providing a valuable resource for practicing clinicians. In addition to the full guidelines, ESC offers the ESC Pocket Guidelines App and printed Pocket Guidelines. All ESC guidelines can be accessed at the following link: http://www.escardio.org/Guidelines-&-Education/Clinical-Practice-Guidelines.
- © 2015 SAGE Publications