Summary
This article focuses on stenting within clinical trials. Topics include results from the ABSORB trial, novel stent designs, stent thrombosis, and results of a second-generation stent evaluated in the SPIRIT III trial, among other things.
- Interventional Techniques & Devices Clinical Trials
- Thrombotic Disorders
First Bioabsorbable Stent Looks Promising
The 6-month results of the ABSORB trial demonstrated the safety and efficacy of the first fully bioabsorbable drug-eluting stent (DES) to be evaluated in a clinical trial. PW Serruys, MD, PhD, of Erasmus Medical Center, Rotterdam, The Netherlands, reported the early results in 24 patients with a single lesion, treated in Europe and New Zealand with the Bioabsorbable Everolimus-Eluting Coronary Stent System (BVS EECSS).
At 6 months, the major adverse cardiac events (MACE) rate was 3.3% (1 patient with non-Q-wave myocardial infection [MI]). In-stent late loss was 0.44 mm, possibly driven by bioactive remodeling or mechanical late recoil “which is being addressed by a modification of the stent design,” Dr. Serruys said, noting that this falls in between the 0.85 mm late loss with bare metal stents (BMS) and 0.10 to 0.20 with other types of DES. Stent area was 6.08 mm2 post-PCI and 5.37 mm2 at 6 months, for a difference of −11.7 %. Restenosis rate was 11.5%, and stent area obstruction was 5.5%.
Capturing Endothelial Progenitor Cells: Novel Stent Design Looks Good
Strong initial results were shown for the Genous Endothelial Progenitor Cell (EPC)-capturing stent, which is coated with the CD34+ antibody and attracts EPCs. The EPCs then differentiate into a functional endothelial layer that is believed to accelerate the healing process and thus prevent in-stent restenosis and possibly stent-related thrombosis. A single-center study reported by Marcel Beijk, MD, of the University of Amsterdam, The Netherlands, involved 87 patients treated with PCI (after 2 weeks on a statin) using the EPC-capturing stent. Procedural success (99.3% TIMI 3 flow post PCI) and clinical outcomes at 6 months (target vessel revascularization 3.7%) were both very favorable. Dr. Beijk commented, “This is still an early study, but it offers a whole new look at the use of stents and the process of healing damaged tissue. We believe this is a step in the right direction.”
Stent Thrombosis in the “Real World”
A report from Denmark offered a look at the “real world” of stenting in patients who receive the recommended 12 months of dual antiplatelet therapy. The study involved 8,847 patients who received BMS (11,730 stents) and 3,548 who received DES (5,422 stents), followed for at least 15 months. The analysis found no overall differences in stent thrombosis, MI, and mortality between the two stent groups. Mortality was 6.2% with BMS and 4.4% with DES, while stent thrombosis was 0.61% and 0.65% with BMS and DES respectively, reported Michael Maeng, MD, of the Aarhus University Hospital, Skejby, Denmark.
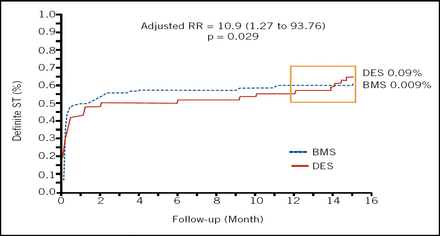
Two differences did emerge, however; a 43% adjusted relative reduction in TVR favoring DES, and a small, but highly significant, excess of definite stent thrombosis and MI in the DES group 12 to 15 months after the index PCI (Figure 1).
Definite Stent Thrombosis at 12–15 Months After Index PCI.
While 15 months may be an insufficient time for fully quantifying risks, at this point Dr. Maeng maintained “the minor risk of very late stent thrombosis and MI does not outweigh the benefit of DES.”
Second-Generation Stent Shines
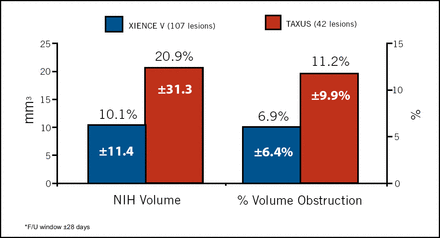
The SPIRIT III trial evaluated the noninferiority of the everolimus-eluting XIENCE V stent versus the paclitaxel-eluting TAXUS stent in 1,002 patients enrolled at 65 US sites, and serves as the pivotal approval study for XIENCE V. Greg W. Stone, MD, Columbia University Medical Center, New York, reported that XIENCE V was superior in reducing in-segment late loss, the primary endpoint of the trial. XIENCE V significantly reduced in-stent late loss at 8 months (from 0.28 with TAXUS to 0.14 with XIENCE V) and reduced follow-up diameter stenosis (from 22.8% to 18.8%), with a trend towards lower binary restenosis and a significant reduction in in-stent volume obstruction without late acquired malapposition (Figure 2).
IVUS In-Stent Measures at 8 Months.
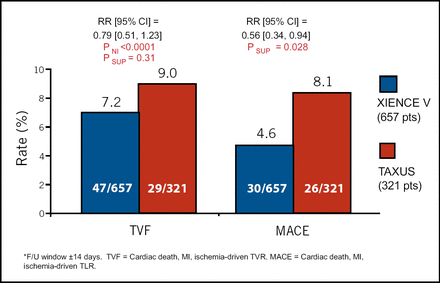
Target vessel failure rates were similar, 7.2% with XIENCE V and 9.0% with TAXUS, with a trend observed for fewer TVFs with XIENCE V. Major adverse cardiac events (MACE) were observed in 4.6% and 8.1%, respectively, representing a 44% reduction with XIENCE V (p=0.028; Figure 3). The occurrence of death, MI, and stent thrombosis was similar between the two devices. Dr. Stone concluded, “The SPIRIT III trial demonstrates that the XIENCE V stent decreases angiographic restenosis and improves overall freedom from adverse events, compared with the previously most widely used DES.”
TVF and MACE Through 270 days*.
Thrombin Receptor Antagonist: New Protection in PCI?
A novel thrombin receptor antagonist (TRA), SCH 530348, designed for the prevention and treatment of atherothrombosis, was found to improve adverse event rates without increasing the risk of bleeding in a study of 1,030 patients from North America and Europe. The study randomly assigned patients 3:1 to a single loading dose of SCH 530348 (10 to 40 mg) or placebo, after which 573 patients underwent PCI and were randomly assigned to a maintenance dose of 0.5 mg, 1.0 mg, 2.5 mg, or placebo (if they received the placebo loading dose) for 60 days. Of the remaining patients, 75 underwent coronary artery bypass grafting (CABG) and 382 received medical management.
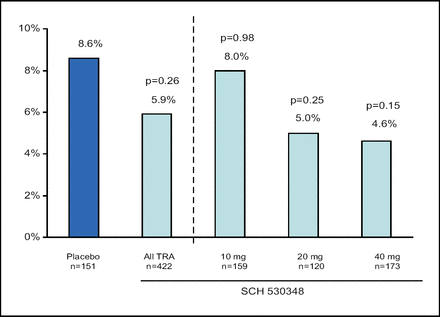
David Moliterno, MD, of the Gill Heart Institute at the University of Kentucky, Lexington, reported that the TRA was associated with an overall non-significant 32% reduction in death or MACE at 60 days (p=0.26), and a 46% reduction at the highest loading dose (40 mg) (p=0.15; Figure 4). The rate of MI was reduced by 41% in the overall TRA group and by 52% with the highest dose (neither change was significant).
PCI Cohort. 60-Day Death or MACE.
TRA and placebo were similar in terms of major or minor bleeding and treatment discontinuation rates. The 40-mg loading dose achieved ≥80% inhibition of platelet aggregation (IPA) in 1 and 2 hours in 68% to 96% of subjects, respectively. Maintenance doses of 1 mg and 2.5 mg sustained ≥=80% IPA at 30 and 60 days in all subjects.
“Thrombin receptor antagonist therapy has the potential to be transformational antiplatelet therapy in the treatment of atherothrombosis, and large phase 3 trials are warranted,” Dr. Moliterno concluded.
- © 2007 MD Conference Express