Summary
Infliximab was the first anti—tumor necrosis factor a monoclonal antibody approved for the treatment of rheumatoid arthritis (RA). In 2013, CT-P13 was the first infliximab biosimilar agent approved by the European Commission. This Phase 3 trial of BOW-015 is the first study to compare an infliximab biosimilar agent with infliximab in patients with RA, assessing time points before Week 14.
- Rheumatology Clinical Trials
- Rheumatoid Arthritis
- Rheumatology Clinical Trials
- Rheumatology
- Rheumatoid Arthritis
Infliximab was the first anti–tumor necrosis factor α monoclonal antibody approved for the treatment of rheumatoid arthritis (RA). In 2013, CT-P13 was the first infliximab biosimilar agent approved by the European Commission. This Phase 3 trial of BOW-015, presented by Jonathan Kay, MD, University of Massachusetts Medical School, Worcester, Massachusetts, USA, is the first study to compare an infliximab biosimilar agent with infliximab in patients with RA, assessing time points before Week 14.
The primary objective of this prospective randomized double-blind study was to determine the equivalence of BOW-015 with reference infliximab during 16 weeks of treatment. The key secondary objectives were to assess the long-term efficacy, safety, and tolerability of BOW-015 and determine BOW-015 serum concentrations and immunogenicity.
Patients with RA were randomly assigned to treatment with BOW-015 (n=127) or infliximab (n=62). The primary endpoint, American College of Rheumatology (ACR) 20 response rate, was assessed at Week 16. A total of 161 responders from both groups received open-label BOW-015 until Week 58. Nonresponders were followed to Week 26 (n=20).
Patients were included if they were aged 18 to 65 years with RA for ≥2 years and on stable medication doses, including methotrexate. Those who previously were treated with biological agents or who had active tuberculosis or evidence of latent tuberculosis were excluded. Treatment was completed by 120 patients (94%) in the BOW-015 group and 61 patients (98%) in the infliximab group. Baseline characteristics were similar between the 2 groups.
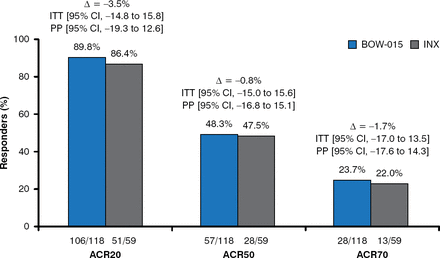
At Week 16, there were no significant differences in ACR response rates between the BOW-015 and infliximab groups. ACR20 response rates were 89.8% in the BOW-015 group, compared with 86.4% in the infliximab group (intention-to-treat [ITT] 95% CI, −14.8 to 15.8; per-protocol [PP] 95% CI, −19.3 to 12.6). ACR50 response rates were 48.3% in the BOW-015 group versus 47.5% in the infliximab group (ITT 95% CI, −15.0 to 15.6; PP 95% CI, −16.8 to 15.1). ACR70 response rates were 23.7% in the BOW-015 group, compared with 22.0% in the infliximab group (ITT 95% CI, −17.0 to 13.5; PP 95% CI, −17.6 to 14.3; Figure 1).
ACR Response Rates at Week 16
ACR=American College of Rheumatology; INX=infliximab; ITT=intention to treat;
PP=per protocol.
Reproduced with permission from J Kay, MD.
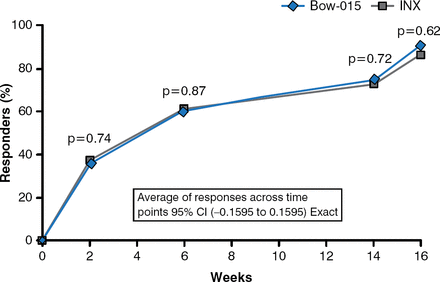
The ACR20 response rates of patients in the BOW-015 and infliximab groups were almost the same at all assessments over 16 weeks (Figure 2).
ACR20 Response Rates Over 16 Weeks (Per-Protocol Analysis)
INX=infliximab.
Reproduced with permission from J Kay, MD.
At Week 16, there were no significant differences in adverse event rates between the BOW-015 group (43%) and the infliximab group (50%; p=0.44). Tuberculosis infection was reported in 3 patients (2%) in the BOW-015 group compared with none in the infliximab group (p=0.55). Five patients (4%) in the BOW-015 group and 1 (1%) in the infliximab group discontinued the study drug due to adverse events (p=0.67).
At Week 16, the efficacy of BOW-015 and infliximab were similar and within the prespecified clinical equivalence margin. The incidence of treatment-emergent adverse events and serious adverse events was similar between the 2 groups. Rates of tuberculosis infection were lower than expected in this study population. The results of the open-label phase—including 1-year immunogenicity, safety, and long-term responder rates—will be available in fall 2014.
- © 2014 MD Conference Express®